Muscle ring finger protein-1 inhibits PKC{epsilon} activation and prevents cardiomyocyte hypertrophy
- PMID: 15596539
- PMCID: PMC2172633
- DOI: 10.1083/jcb.200402033
Muscle ring finger protein-1 inhibits PKC{epsilon} activation and prevents cardiomyocyte hypertrophy
Abstract
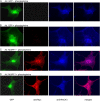
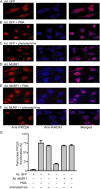
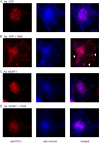
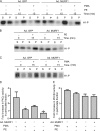
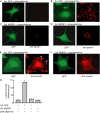
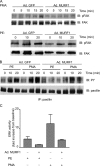
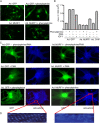
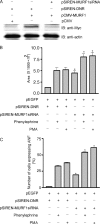
Much effort has focused on characterizing the signal transduction cascades that are associated with cardiac hypertrophy. In spite of this, we still know little about the mechanisms that inhibit hypertrophic growth. We define a novel anti-hypertrophic signaling pathway regulated by muscle ring finger protein-1 (MURF1) that inhibits the agonist-stimulated PKC-mediated signaling response in neonatal rat ventricular myocytes. MURF1 interacts with receptor for activated protein kinase C (RACK1) and colocalizes with RACK1 after activation with phenylephrine or PMA. Coincident with this agonist-stimulated interaction, MURF1 blocks PKCepsilon translocation to focal adhesions, which is a critical event in the hypertrophic signaling cascade. MURF1 inhibits focal adhesion formation, and the activity of downstream effector ERK1/2 is also inhibited in the presence of MURF1. MURF1 inhibits phenylephrine-induced (but not IGF-1-induced) increases in cell size. These findings establish that MURF1 is a key regulator of the PKC-dependent hypertrophic response and can blunt cardiomyocyte hypertrophy, which may have important implications in the pathophysiology of clinical cardiac hypertrophy.
Figures


Similar articles
-
Muscle RING finger-1 attenuates IGF-I-dependent cardiomyocyte hypertrophy by inhibiting JNK signaling.Am J Physiol Endocrinol Metab. 2014 Apr 1;306(7):E723-39. doi: 10.1152/ajpendo.00326.2013. Epub 2014 Jan 14. Am J Physiol Endocrinol Metab. 2014. PMID: 24425758 Free PMC article.
-
Activation of AMPK inhibits cardiomyocyte hypertrophy by modulating of the FOXO1/MuRF1 signaling pathway in vitro.Acta Pharmacol Sin. 2010 Jul;31(7):798-804. doi: 10.1038/aps.2010.73. Epub 2010 Jun 28. Acta Pharmacol Sin. 2010. PMID: 20581852 Free PMC article.
-
PICOT attenuates cardiac hypertrophy by disrupting calcineurin-NFAT signaling.Circ Res. 2008 Mar 28;102(6):711-9. doi: 10.1161/CIRCRESAHA.107.165985. Epub 2008 Feb 7. Circ Res. 2008. PMID: 18258855
-
Cardiac-specific overexpression of diacylglycerol kinase zeta prevents Gq protein-coupled receptor agonist-induced cardiac hypertrophy in transgenic mice.Circulation. 2006 Jan 3;113(1):60-6. doi: 10.1161/CIRCULATIONAHA.105.560771. Epub 2005 Dec 27. Circulation. 2006. PMID: 16380548
-
PRKCE gene encoding protein kinase C-epsilon-Dual roles at sarcomeres and mitochondria in cardiomyocytes.Gene. 2016 Sep 15;590(1):90-6. doi: 10.1016/j.gene.2016.06.016. Epub 2016 Jun 13. Gene. 2016. PMID: 27312950 Free PMC article. Review.
Cited by
-
MuRF1/TRIM63, Master Regulator of Muscle Mass.Int J Mol Sci. 2020 Sep 11;21(18):6663. doi: 10.3390/ijms21186663. Int J Mol Sci. 2020. PMID: 32933049 Free PMC article. Review.
-
Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction.Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4377-82. doi: 10.1073/pnas.0611726104. Epub 2007 Mar 2. Proc Natl Acad Sci U S A. 2007. PMID: 17360532 Free PMC article.
-
Heart failure-specific changes in protein kinase signalling.Pflugers Arch. 2014 Jun;466(6):1151-62. doi: 10.1007/s00424-014-1462-x. Epub 2014 Feb 8. Pflugers Arch. 2014. PMID: 24510065 Review.
-
A muscle-specific MuRF1-E2 network requires stabilization of MuRF1-E2 complexes by telethonin, a newly identified substrate.J Cachexia Sarcopenia Muscle. 2018 Feb;9(1):129-145. doi: 10.1002/jcsm.12249. Epub 2017 Dec 22. J Cachexia Sarcopenia Muscle. 2018. PMID: 29271608 Free PMC article.
-
Paracrine effects of IGF-1 overexpression on the functional decline due to skeletal muscle disuse: molecular and functional evaluation in hindlimb unloaded MLC/mIgf-1 transgenic mice.PLoS One. 2013 Jun 3;8(6):e65167. doi: 10.1371/journal.pone.0065167. Print 2014. PLoS One. 2013. PMID: 23755187 Free PMC article.
References
-
- Allo, S.N., L.L. Carl, and H.E. Morgan. 1992. Acceleration of growth of cultured cardiomyocytes and translocation of protein kinase C. Am. J. Physiol. 263:C319–C325. - PubMed
-
- Besson, A., T.L. Wilson, and V.W. Yong. 2002. The anchoring protein RACK1 links protein kinase Cε to integrin β chains. Requirement for adhesion and motility. J. Biol. Chem. 277:22073–22084. - PubMed
-
- Bodine, S.C., E. Latres, S. Baumhueter, V.K. Lai, L. Nunez, B.A. Clarke, W.T. Poueymirou, F.J. Panaro, E. Na, K. Dharmarajan, et al. 2001. a. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 294:1704–1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

