Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42
- PMID: 15596540
- PMCID: PMC2172605
- DOI: 10.1083/jcb.200403043
Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42
Abstract
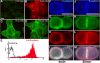
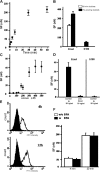
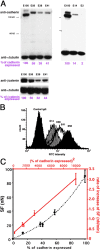
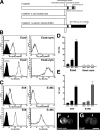
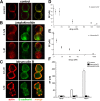
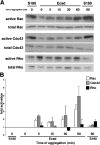
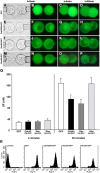
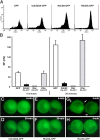
We have used a modified, dual pipette assay to quantify the strength of cadherin-dependent cell-cell adhesion. The force required to separate E-cadherin-expressing paired cells in suspension was measured as an index of intercellular adhesion. Separation force depended on the homophilic interaction of functional cadherins at the cell surface, increasing with the duration of contact and with cadherin levels. Severing the link between cadherin and the actin cytoskeleton or disrupting actin polymerization did not affect initiation of cadherin-mediated adhesion, but prevented it from developing and becoming stronger over time. Rac and Cdc42, the Rho-like small GTPases, were activated when E-cadherin-expressing cells formed aggregates in suspension. Overproduction of the dominant negative form of Rac or Cdc42 permitted initial E-cadherin-based adhesion but affected its later development; the dominant active forms prevented cell adhesion outright. Our findings highlight the crucial roles played by Rac, Cdc42, and actin cytoskeleton dynamics in the development and regulation of strong cell adhesion, defined in terms of mechanical forces.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

