Quantitative mass spectrometry reveals a role for the GTPase Rho1p in actin organization on the peroxisome membrane
- PMID: 15596542
- PMCID: PMC2172632
- DOI: 10.1083/jcb.200404119
Quantitative mass spectrometry reveals a role for the GTPase Rho1p in actin organization on the peroxisome membrane
Abstract
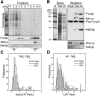
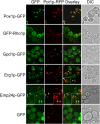
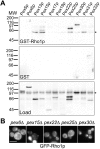
We have combined classical subcellular fractionation with large-scale quantitative mass spectrometry to identify proteins that enrich specifically with peroxisomes of Saccharomyces cerevisiae. In two complementary experiments, isotope-coded affinity tags and tandem mass spectrometry were used to quantify the relative enrichment of proteins during the purification of peroxisomes. Mathematical modeling of the data from 306 quantified proteins led to a prioritized list of 70 candidates whose enrichment scores indicated a high likelihood of them being peroxisomal. Among these proteins, eight novel peroxisome-associated proteins were identified. The top novel peroxisomal candidate was the small GTPase Rho1p. Although Rho1p has been shown to be tethered to membranes of the secretory pathway, we show that it is specifically recruited to peroxisomes upon their induction in a process dependent on its interaction with the peroxisome membrane protein Pex25p. Rho1p regulates the assembly state of actin on the peroxisome membrane, thereby controlling peroxisome membrane dynamics and biogenesis.
Figures






Similar articles
-
Pex11-related proteins in peroxisome dynamics: a role for the novel peroxin Pex27p in controlling peroxisome size and number in Saccharomyces cerevisiae.Mol Biol Cell. 2003 Oct;14(10):4089-102. doi: 10.1091/mbc.e03-03-0150. Epub 2003 May 18. Mol Biol Cell. 2003. PMID: 14517321 Free PMC article.
-
Expression of the Salmonella spp. virulence factor SifA in yeast alters Rho1 activity on peroxisomes.Mol Biol Cell. 2010 Oct 15;21(20):3567-77. doi: 10.1091/mbc.E10-06-0482. Epub 2010 Aug 25. Mol Biol Cell. 2010. PMID: 20739463 Free PMC article.
-
A dual function for Pex3p in peroxisome formation and inheritance.J Cell Biol. 2009 Nov 16;187(4):463-71. doi: 10.1083/jcb.200906161. Epub 2009 Nov 9. J Cell Biol. 2009. PMID: 19948495 Free PMC article.
-
Peroxisome membrane biogenesis: the stage is set.Curr Biol. 2004 May 25;14(10):R397-9. doi: 10.1016/j.cub.2004.05.017. Curr Biol. 2004. PMID: 15186768 Review.
-
Peroxisome biogenesis: advances and conundrums.Curr Opin Cell Biol. 2003 Aug;15(4):489-97. doi: 10.1016/s0955-0674(03)00082-6. Curr Opin Cell Biol. 2003. PMID: 12892791 Review.
Cited by
-
Peroxins Pex30 and Pex29 Dynamically Associate with Reticulons to Regulate Peroxisome Biogenesis from the Endoplasmic Reticulum.J Biol Chem. 2016 Jul 22;291(30):15408-27. doi: 10.1074/jbc.M116.728154. Epub 2016 Apr 29. J Biol Chem. 2016. PMID: 27129769 Free PMC article.
-
Peroxisome biogenesis and inter-organelle communication: an indispensable role for Pex11 and Pex30 family proteins in yeast.Curr Genet. 2022 Dec;68(5-6):537-550. doi: 10.1007/s00294-022-01254-y. Epub 2022 Oct 15. Curr Genet. 2022. PMID: 36242632 Review.
-
Peroxisomes take shape.Nat Rev Mol Cell Biol. 2013 Dec;14(12):803-17. doi: 10.1038/nrm3700. Nat Rev Mol Cell Biol. 2013. PMID: 24263361 Free PMC article. Review.
-
A Drosophila model for the Zellweger spectrum of peroxisome biogenesis disorders.Dis Model Mech. 2011 Sep;4(5):659-72. doi: 10.1242/dmm.007419. Epub 2011 Jun 13. Dis Model Mech. 2011. PMID: 21669930 Free PMC article.
-
Expression and functional profiling reveal distinct gene classes involved in fatty acid metabolism.Mol Syst Biol. 2006;2:2006.0009. doi: 10.1038/msb4100051. Epub 2006 Feb 21. Mol Syst Biol. 2006. PMID: 16738555 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

