The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets
- PMID: 15596543
- PMCID: PMC2172618
- DOI: 10.1083/jcb.200405148
The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets
Abstract
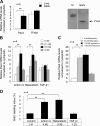
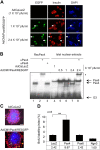
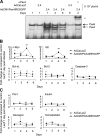
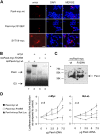
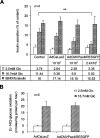
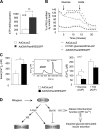
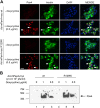
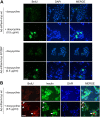
The mechanism by which the beta-cell transcription factor Pax4 influences cell function/mass was studied in rat and human islets of Langerhans. Pax4 transcripts were detected in adult rat islets, and levels were induced by the mitogens activin A and betacellulin. Wortmannin suppressed betacellulin-induced Pax4 expression, implicating the phosphatidylinositol 3-kinase signaling pathway. Adenoviral overexpression of Pax4 caused a 3.5-fold increase in beta-cell proliferation with a concomitant 1.9-, 4-, and 5-fold increase in Bcl-xL (antiapoptotic), c-myc, and Id2 mRNA levels, respectively. Accordingly, Pax4 transactivated the Bcl-xL and c-myc promoters, whereas its diabetes-linked mutant was less efficient. Bcl-xL activity resulted in altered mitochondrial calcium levels and ATP production, explaining impaired glucose-induced insulin secretion in transduced islets. Infection of human islets with an inducible adenoviral Pax4 construct caused proliferation and protection against cytokine-evoked apoptosis, whereas the mutant was less effective. We propose that Pax4 is implicated in beta-cell plasticity through the activation of c-myc and potentially protected from apoptosis through Bcl-xL gene expression.
Figures
Similar articles
-
The diabetes-linked transcription factor Pax4 is expressed in human pancreatic islets and is activated by mitogens and GLP-1.Hum Mol Genet. 2008 Feb 15;17(4):478-89. doi: 10.1093/hmg/ddm325. Epub 2007 Nov 7. Hum Mol Genet. 2008. PMID: 17989064
-
In vivo conditional Pax4 overexpression in mature islet β-cells prevents stress-induced hyperglycemia in mice.Diabetes. 2011 Jun;60(6):1705-15. doi: 10.2337/db10-1102. Epub 2011 Apr 26. Diabetes. 2011. PMID: 21521872 Free PMC article.
-
The transcription factor PAX4 acts as a survival gene in INS-1E insulinoma cells.Oncogene. 2007 Jun 21;26(29):4261-71. doi: 10.1038/sj.onc.1210205. Epub 2007 Jan 29. Oncogene. 2007. PMID: 17260022
-
Pax4 in Health and Diabetes.Int J Mol Sci. 2023 May 5;24(9):8283. doi: 10.3390/ijms24098283. Int J Mol Sci. 2023. PMID: 37175989 Free PMC article. Review.
-
The gene Pax4 is an essential regulator of pancreatic beta-cell development.Mol Cells. 2004 Dec 31;18(3):289-94. Mol Cells. 2004. PMID: 15650323 Review.
Cited by
-
Development of a reliable automated screening system to identify small molecules and biologics that promote human β-cell regeneration.Am J Physiol Endocrinol Metab. 2016 Nov 1;311(5):E859-E868. doi: 10.1152/ajpendo.00515.2015. Epub 2016 Sep 13. Am J Physiol Endocrinol Metab. 2016. PMID: 27624103 Free PMC article.
-
Pax4 is not essential for beta-cell differentiation in zebrafish embryos but modulates alpha-cell generation by repressing arx gene expression.BMC Dev Biol. 2012 Dec 17;12:37. doi: 10.1186/1471-213X-12-37. BMC Dev Biol. 2012. PMID: 23244389 Free PMC article.
-
Transcriptional response of pancreatic beta cells to metabolic stimulation: large scale identification of immediate-early and secondary response genes.BMC Mol Biol. 2007 Jun 22;8:54. doi: 10.1186/1471-2199-8-54. BMC Mol Biol. 2007. PMID: 17587450 Free PMC article.
-
Inhibition of beta cell growth and function by bone morphogenetic proteins.Diabetologia. 2014 Dec;57(12):2546-54. doi: 10.1007/s00125-014-3384-8. Epub 2014 Sep 27. Diabetologia. 2014. PMID: 25260823
-
Knowledge Gaps in Rodent Pancreas Biology: Taking Human Pluripotent Stem Cell-Derived Pancreatic Beta Cells into Our Own Hands.Front Endocrinol (Lausanne). 2016 Jan 14;6:194. doi: 10.3389/fendo.2015.00194. eCollection 2015. Front Endocrinol (Lausanne). 2016. PMID: 26834702 Free PMC article. Review.
References
-
- Baumann Kubetzko, F.B., C. Di Paolo, C. Maag, R. Meier, B.W. Schafer, D.R. Betts, R.A. Stahel, and A. Himmelmann. 2004. The PAX5 oncogene is expressed in N-type neuroblastoma cells and increases tumorigenicity of a S-type cell line. Carcinogenesis. 25:1839–1846. - PubMed
-
- Bonner-Weir, S. 2000. Life and death of the pancreatic beta cells. Trends Endocrinol. Metab. 11:375–378. - PubMed
-
- Buteau, J., S. Foisy, C.J. Rhodes, L. Carpenter, T.J. Biden, and M. Prentki. 2001. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes. 50:2237–2243. - PubMed
-
- Buteau, J., S. Foisy, E. Joly, and M. Prentki. 2003. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 52:124–132. - PubMed
-
- Campbell, S.C., H. Cragg, L.J. Elrick, W.M. Macfarlane, K.I. Shennan, and K. Docherty. 1999. Inhibitory effect of Pax4 on the human insulin and islet amyloid polypeptide (IAPP) promoters. FEBS Lett. 463:53–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

