Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease
- PMID: 15596606
- PMCID: PMC1482312
- DOI: 10.1001/archneur.61.12.1859
Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease
Abstract
Background: The amyloid-beta (Abeta) peptide has a central role in the neurodegeneration of Alzheimer disease (AD). Immunization of AD transgenic mice with Abeta(1-42) (Abeta(42)) peptide reduces both the spatial memory impairments and AD-like neuropathologic changes in these mice. Therapeutic immunization with Abeta in patients with AD was shown to be effective in reducing Abeta deposition, but studies were discontinued owing to the development of an autoimmune, cell-mediated meningoencephalitis. We hypothesized that gene vaccination could be used to generate an immune response to Abeta(42) that produced antibody response but avoided an adverse cell-mediated immune effect.
Objective: To develop an effective genetic immunization approach for treatment and prevention of AD without causing an autoimmune, cell-mediated meningoencephalitis.
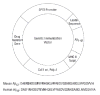
Methods: Mice were vaccinated with a plasmid that encodes Abeta(42), administered by gene gun. The immune response of the mice to Abeta(42) was monitored by measurement of (1) antibody levels by enzyme-linked immunosorbent assay (ELISA) and Western blot and (2) Abeta(42)-specific T-cell response as measured by interferon-gamma enzyme-linked immunospot (ELISPOT) assay.
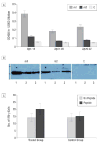
Results: Gene-gun delivery of the mouse Abeta(42) dimer gene induced significant humoral immune responses in BALB/c wild-type mice after 3 vaccinations in 10-day intervals. All 3 mice in the treated group showed significant humoral immune responses. The ELISPOT assay for interferon-gamma release with mouse Abeta(42) peptide and Abeta(9-18) showed no evident cytotoxic T-lymphocyte response. We further tested the responses of wild-type BALB/c mice to the monomer Abeta(42) gene vaccine. Western blot evaluation showed both human and mouse Abeta monomer gene vaccine elicited detectable humoral immune responses. We also introduced the human Abeta(42) monomer gene vaccine into AD double transgenic mice APPswe/PSEN1(A246E). Mice were vaccinated with plasmids that encode Abeta(1-42) and Abeta(1-16), or with plasmid without the Abeta gene. Treated mice showed significant humoral immune responses as demonstrated by ELISA and by Western blot. These mice also showed no significant cellular immune response as tested by ELISPOT. One of the treated mice was killed at 7 months of age for histological observations, and scattered amyloid plaques were noted in all layers of the cerebral cortex and in the hippocampus in both Abeta(42)- and control-vaccinated mice. No definite difference was discerned between the experimental and control animals.
Conclusions: Gene-gun-administered genetic immunization with the Abeta(42) gene in wild-type BALB/c and AD transgenic mice can effectively elicit humoral immune responses without a significant T-cell-mediated immune response to the Abeta peptide. This immunotherapeutic approach could provide an alternative active immunization method for therapy and prevention of AD.
Figures


Comment in
-
DNA vaccination may open up a new avenue for treatment of Alzheimer disease.Arch Neurol. 2004 Dec;61(12):1832. doi: 10.1001/archneur.61.12.1832. Arch Neurol. 2004. PMID: 15596601 No abstract available.
Similar articles
-
Amyloid β 3-10 DNA vaccination suggests a potential new treatment for Alzheimer's disease in BALB/c mice.Chin Med J (Engl). 2011 Sep;124(17):2636-41. Chin Med J (Engl). 2011. PMID: 22040416
-
Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology.Alzheimers Res Ther. 2018 Nov 20;10(1):115. doi: 10.1186/s13195-018-0441-4. Alzheimers Res Ther. 2018. PMID: 30454039 Free PMC article.
-
Immunization with a new DNA vaccine for Alzheimer's disease elicited Th2 immune response in BALB/c mice by in vivo electroporation.J Neurol Sci. 2012 Feb 15;313(1-2):17-21. doi: 10.1016/j.jns.2011.09.040. Epub 2011 Oct 24. J Neurol Sci. 2012. PMID: 22029939
-
[Immunotherapy of Alzheimer's disease. Results of experimental investigations and treatment of perspectives].Fortschr Neurol Psychiatr. 2004 Apr;72(4):204-19. doi: 10.1055/s-2004-818390. Fortschr Neurol Psychiatr. 2004. PMID: 15095177 Review. German.
-
Amyloid-beta immunotherapy for the prevention and treatment of Alzheimer disease: lessons from mice, monkeys, and humans.Rejuvenation Res. 2006 Spring;9(1):77-84. doi: 10.1089/rej.2006.9.77. Rejuvenation Res. 2006. PMID: 16608400 Review.
Cited by
-
The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells.Biomaterials. 2013 Mar;34(9):2359-69. doi: 10.1016/j.biomaterials.2012.11.066. Epub 2012 Dec 27. Biomaterials. 2013. PMID: 23274070 Free PMC article.
-
Anti-amyloid beta to tau - based immunization: Developments in immunotherapy for Alzheimer disease.Immunotargets Ther. 2013 Aug 1;2013(2):105-114. doi: 10.2147/ITT.S31428. Immunotargets Ther. 2013. PMID: 24926455 Free PMC article.
-
In vivo electroporation of a new gene vaccine encoding ten repeats of Aβ3-10 prevents brain Aβ deposition and delays cognitive impairment in young Tg-APPswe/PSEN1dE9 mice.Neurochem Res. 2012 Jul;37(7):1534-44. doi: 10.1007/s11064-012-0748-7. Epub 2012 Mar 22. Neurochem Res. 2012. PMID: 22437434
-
A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Aβ42 DNA vaccine for Alzheimer disease.J Neuroimmunol. 2013 Jan 15;254(1-2):63-8. doi: 10.1016/j.jneuroim.2012.09.008. Epub 2012 Oct 1. J Neuroimmunol. 2013. PMID: 23036592 Free PMC article.
-
Translational research on the way to effective therapy for Alzheimer disease.Arch Gen Psychiatry. 2005 Nov;62(11):1186-92. doi: 10.1001/archpsyc.62.11.1186. Arch Gen Psychiatry. 2005. PMID: 16275806 Free PMC article. Review.
References
-
- Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease: hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid β-protein. Ann N Y Acad Sci. 2000;924:17–25. - PubMed
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Hardy J. Amyloid, the preseniline and Alzheimer’s disease. Trends Neurosci. 1997;20:154–159. - PubMed
-
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399(6738 suppl):A23–A31. - PubMed
-
- Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer’s disease. Science. 2003;302:834–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

