gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis
- PMID: 15596715
- PMCID: PMC535585
- DOI: 10.1073/pnas.0408340102
gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis
Abstract
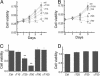
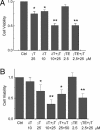
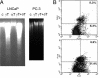
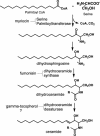
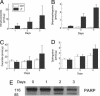
gamma-Tocopherol (gammaT), the predominant form of vitamin E in diets, but not alpha-tocopherol, the major vitamin E form in tissues and supplements, inhibits proliferation of prostate cancer cells (LNCaP and PC-3) and lung cancer cells (A549). In contrast, at similar concentrations, gammaT has no effect on normal prostate epithelial cells. Combinations of some vitamin E forms, such as gammaT and delta-tocopherol, exhibit additive or synergistic inhibitory effects. In this study, gammaT or its combination with delta-tocopherol induced apoptosis in androgen-sensitive prostate LNCaP, but not in androgen-resistant PC-3 cells, by the induction of cytochrome c release, activation of caspase 9 and caspase 3, cleavage of poly-ADP-ribose polymerase (PARP), and involvement of caspase-independent pathways. Myriocin and fumonisin B1, specific inhibitors of key enzymes (serine palmitoyltransferase and dihydroceramide synthase, respectively) in de novo synthesis of sphingolipids, significantly protected cells from gammaT-induced DNA fragmentation, cytochrome c release, PARP cleavage, and the formation of active caspase 3. Compared with vehicle-treated controls, gammaT treatment led to pronounced dihydroceramide and dihydrosphingosine accumulation, which preceded morphological and biochemical manifestations of apoptosis. In contrast, ceramide and shpingosine levels did not increase until day 3, when substantial cell death took place. Our study demonstrates that gammaT and mixed vitamin E forms induce cell death by interrupting the de novo sphingolipid pathway in a prostate cancer cell line. Thus, certain vitamin E forms may be valuable as anticancer agents.
Figures
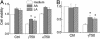



References
-
- Eichholzer, M., Stahelin, H. B., Gey, K. F., Ludin, E. & Bernasconi, F. (1996) Int. J. Cancer 66, 145-150. - PubMed
-
- Toth, B. & Patil, K. (1983) J. Natl. Cancer Inst. 70, 1107-1111. - PubMed
-
- Chung, H., Wu, D., Han, S. N., Gay, R., Goldin, B., Bronson, R. E., Mason, J. B., Smith, D. E. & Meydani, S. N. (2003) J. Nutr. 133, 528-532. - PubMed
-
- Chan, J. M., Stampfer, M. J., Ma, J., Rimm, E. B., Willett, W. C. & Giovannucci, E. L. (1999) Cancer Epidemiol. Biomarkers Prev. 8, 893-899. - PubMed
-
- Moyad, M. A. (2002) Urology 59, 9-19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

