Identification of an adeno-associated virus Rep protein binding site in the adenovirus E2a promoter
- PMID: 15596798
- PMCID: PMC538739
- DOI: 10.1128/JVI.79.1.28-38.2005
Identification of an adeno-associated virus Rep protein binding site in the adenovirus E2a promoter
Abstract
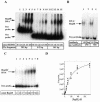
Adeno-associated virus (AAV) and other parvoviruses inhibit proliferation of nonpermissive cells. The mechanism of this inhibition is not thoroughly understood. To learn how AAV interacts with host cells, we investigated AAV's interaction with adenovirus (Ad), AAV's most efficient helper virus. Coinfection with Ad and AAV results in an AAV-mediated inhibition of Ad5 gene expression and replication. The AAV replication proteins (Rep) activate and repress gene expression from AAV and heterologous transcription promoters. To investigate the role of Rep proteins in the suppression of Ad propagation, we performed chromatin immunoprecipitation analyses that demonstrated in vivo AAV Rep protein interaction with the Ad E2a gene promoter. In vitro binding of purified AAV Rep68 protein to the Ad E2a promoter was characterized by electrophoretic mobility shift assays (Kd= 200 +/- 25 nM). A 38 bp, Rep68-protected region (5'-TAAGAGTCAGCGCGCAGTATTTACTGAAGAGAGCCT-3') was identified by DNase I footprint analysis. The 38-bp protected region contains the weak E2a TATA box, sequence elements that resemble the Rep binding sites identified by random sequence oligonucleotide selection, and the transcription start site. These results suggest that Rep binding to the E2a promoter contributes to the inhibition of E2a gene expression from the Ad E2a promoter and may affect Ad replication.
Figures





References
-
- Bantel-Schaal, U., and H. zur Hausen. 1988. Adeno-associated viruses inhibit SV40 DNA amplification and replication of herpes simplex virus in SV40-transformed hamster cells. Virology 164:64-74. - PubMed
-
- Batchu, R. B., and P. L. Hermonat. 1995. The trans-inhibitory Rep78 protein of adeno-associated virus binds to TAR region DNA of the human immunodeficiency virus type 1 long terminal repeat. FEBS Lett. 367:267-271. - PubMed
-
- Batchu, R. B., M. A. Shammas, J. Y. Wang, and N. C. Munshi. 2001. Dual level inhibition of E2F-1 activity by adeno-associated virus Rep78. J. Biol. Chem. 276:24315-24322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

