Expression and characterization of a soluble, active form of the jaagsiekte sheep retrovirus receptor, Hyal2
- PMID: 15596803
- PMCID: PMC538683
- DOI: 10.1128/JVI.79.1.79-86.2005
Expression and characterization of a soluble, active form of the jaagsiekte sheep retrovirus receptor, Hyal2
Erratum in
- J Virol. 2005 Mar;79(5):3228
-
Correction for Vigdorovich et al., Expression and Characterization of a Soluble, Active Form of the Jaagsiekte Sheep Retrovirus Receptor, Hyal2.J Virol. 2016 Jan 28;90(4):2159. doi: 10.1128/JVI.03001-15. Print 2016 Feb 15. J Virol. 2016. PMID: 26822597 Free PMC article. No abstract available.
Abstract
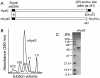
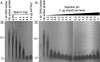
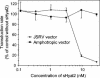
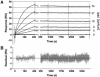
Retrovirus entry into cells is mediated by specific interactions between virus envelope glycoproteins and cell surface receptors. Many of these receptors contain multiple membrane-spanning regions, making their purification and study difficult. The jaagsiekte sheep retrovirus (JSRV) receptor, hyaluronidase 2 (Hyal2), is a glycosylphosphatidylinositol (GPI)-anchored molecule containing no peptide transmembrane regions, making it an attractive candidate for study of retrovirus entry. Further, the hyaluronidase activity reported for human Hyal2, combined with its broad expression pattern, may point to a critical function of Hyal2 in the turnover of hyaluronan, a major extracellular matrix component. Here we describe the properties of a soluble form of human Hyal2 (sHyal2) purified from a baculoviral expression system. sHyal2 is a 54-kDa monomer with weak hyaluronidase activity compared to that of the known hyaluronidase Spam1. In contrast to a previous report indicating that Hyal2 cleaved hyaluronan to a limit product of 20 kDa and was active only at acidic pH, we find that sHyal2 is capable of further degradation of hyaluronan and is active over a broad pH range, consistent with Hyal2 being active at the cell surface where it is normally localized. Interaction of sHyal2 with the JSRV envelope glycoprotein was analyzed by viral inhibition assays, showing >90% inhibition of transduction at 28 nM sHyal2, and by surface plasmon resonance, revealing a remarkably tight specific interaction with a dissociation constant (KD) of 32 +/- 1 pM. In contrast to results obtained with avian retroviruses, purified receptor was not capable of promoting transduction of cells that do not express the virus receptor.
Figures

Similar articles
-
Ability of hyaluronidase 2 to degrade extracellular hyaluronan is not required for its function as a receptor for jaagsiekte sheep retrovirus.J Virol. 2007 Apr;81(7):3124-9. doi: 10.1128/JVI.02177-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229709 Free PMC article.
-
Single residues in the surface subunits of oncogenic sheep retrovirus envelopes distinguish receptor-mediated triggering for fusion at low pH and infection.Virology. 2011 Dec 20;421(2):173-83. doi: 10.1016/j.virol.2011.09.022. Epub 2011 Oct 20. Virology. 2011. PMID: 22018783
-
Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4580-5. doi: 10.1073/pnas.0837136100. Epub 2003 Apr 3. Proc Natl Acad Sci U S A. 2003. PMID: 12676986 Free PMC article.
-
Identification of Hyal2 as the cell-surface receptor for jaagsiekte sheep retrovirus and ovine nasal adenocarcinoma virus.Curr Top Microbiol Immunol. 2003;275:179-99. doi: 10.1007/978-3-642-55638-8_7. Curr Top Microbiol Immunol. 2003. PMID: 12596899 Review.
-
Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein.Oncogene. 2007 Feb 8;26(6):789-801. doi: 10.1038/sj.onc.1209850. Epub 2006 Aug 14. Oncogene. 2007. PMID: 16909114 Review.
Cited by
-
Ability of hyaluronidase 2 to degrade extracellular hyaluronan is not required for its function as a receptor for jaagsiekte sheep retrovirus.J Virol. 2007 Apr;81(7):3124-9. doi: 10.1128/JVI.02177-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229709 Free PMC article.
-
Mouse testicular hyaluronidase-like proteins SPAM1 and HYAL5 but not HYALP1 degrade hyaluronan.Biochem J. 2007 Jan 1;401(1):79-85. doi: 10.1042/BJ20060598. Biochem J. 2007. PMID: 16925524 Free PMC article.
-
Fusogenicity of Jaagsiekte sheep retrovirus envelope protein is dependent on low pH and is enhanced by cytoplasmic tail truncations.J Virol. 2008 Mar;82(5):2543-54. doi: 10.1128/JVI.01852-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094165 Free PMC article.
-
Expression and cellular localization of human hyaluronidase-2 in articular chondrocytes and cultured cell lines.Osteoarthritis Cartilage. 2006 Sep;14(9):849-58. doi: 10.1016/j.joca.2006.02.009. Osteoarthritis Cartilage. 2006. PMID: 16600643 Free PMC article.
-
Receptor binding and low pH coactivate oncogenic retrovirus envelope-mediated fusion.J Virol. 2009 Nov;83(22):11447-55. doi: 10.1128/JVI.00748-09. Epub 2009 Sep 2. J Virol. 2009. PMID: 19726505 Free PMC article.
References
-
- Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

