Improved enzootic nasal tumor virus pseudotype packaging cell lines reveal virus entry requirements in addition to the primary receptor Hyal2
- PMID: 15596804
- PMCID: PMC538734
- DOI: 10.1128/JVI.79.1.87-94.2005
Improved enzootic nasal tumor virus pseudotype packaging cell lines reveal virus entry requirements in addition to the primary receptor Hyal2
Abstract
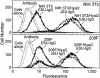
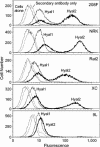
Enzootic nasal tumor virus (ENTV) and jaagsiekte sheep retrovirus (JSRV) are closely related retroviruses that cause epithelial cancers of the respiratory tract in sheep and goats. Both viruses use the glycosylphosphatidylinositol (GPI)-anchored cell surface protein hyaluronidase 2 (Hyal2) as a receptor for cell entry, and entry is mediated by the envelope (Env) proteins encoded by these viruses. Retroviral vectors bearing JSRV Env can transduce cells from a wide range of species, with the exception of rodent cells. Because of the low titer of vectors bearing ENTV Env, it has been difficult to determine the tropism of ENTV vectors, which appeared to transduce cells from sheep and humans only. Here we have developed high-titer ENTV packaging cells and confirm that ENTV has a restricted host range compared to that of JSRV. Most cells that are not transduced by JSRV or ENTV vectors can be made susceptible following expression of human Hyal2 on the cells. However, five rat cell lines from different rat strains and different tissues that were engineered to express human Hyal2 were still only poorly infected by ENTV vectors, even though the ENTV Env protein could bind well to human Hyal2 expressed on four of these cell lines. These results indicate the possibility of a coreceptor requirement for these viruses.
Figures



References
-
- Barker, M., T. Hoshino, O. Gurcay, C. B. Wilson, S. L. Nielsen, R. Downie, and J. Eliason. 1973. Development of an animal brain tumor model and its response to therapy with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 33:976-986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

