Methylation of Tat by PRMT6 regulates human immunodeficiency virus type 1 gene expression
- PMID: 15596808
- PMCID: PMC538702
- DOI: 10.1128/JVI.79.1.124-131.2005
Methylation of Tat by PRMT6 regulates human immunodeficiency virus type 1 gene expression
Abstract
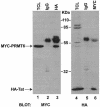
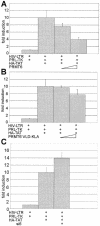
The human immunodeficiency virus (HIV) transactivator protein, Tat, stimulates transcription from the viral long terminal repeats via an arginine-rich transactivating domain. Since arginines are often known to be methylated, we investigated whether HIV type 1 (HIV-1) Tat was a substrate for known protein arginine methyltransferases (PRMTs). Here we identify Tat as a substrate for the arginine methyltransferase, PRMT6. Tat is specifically associated with and methylated by PRMT6 within cells. Overexpression of wild-type PRMT6, but not a methylase-inactive PRMT6 mutant, decreased Tat transactivation of an HIV-1 long terminal repeat luciferase reporter plasmid in a dose-dependent manner. Knocking down PRMT6 consistently increased HIV-1 production in HEK293T cells and also led to increased viral infectiousness as shown in multinuclear activation of a galactosidase indicator assays. Our study demonstrates that arginine methylation of Tat negatively regulates its transactivation activity and that PRMT6 acts as a restriction factor for HIV replication.
Figures



References
-
- An, W., J. Kim, and R. G. Roeder. 2004. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell 117:735-748. - PubMed
-
- Bedford, M. T., A. Frankel, M. B. Yaffe, S. Clarke, P. Leder, and S. Richard. 2000. Arginine methylation inhibits the binding of proline-rich ligands to Src homology 3, but not WW, domains. J. Biol. Chem. 275:16030-16036. - PubMed
-
- Boisvert, F. M., J. Cote, M. C. Boulanger, and S. Richard. 2003. A proteomic analysis of arginine-methylated protein complexes. Mol. Cell Proteomics 2:1319-1330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

