The unique stacked rings in the nucleocapsid of the white spot syndrome virus virion are formed by the major structural protein VP664, the largest viral structural protein ever found
- PMID: 15596810
- PMCID: PMC538705
- DOI: 10.1128/JVI.79.1.140-149.2005
The unique stacked rings in the nucleocapsid of the white spot syndrome virus virion are formed by the major structural protein VP664, the largest viral structural protein ever found
Abstract
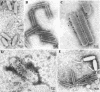
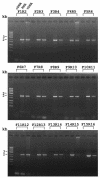
One unique feature of the shrimp white spot syndrome virus (WSSV) genome is the presence of a giant open reading frame (ORF) of 18,234 nucleotides that encodes a long polypeptide of 6,077 amino acids with a hitherto unknown function. In the present study, by applying proteomic methodology to analyze the sodium dodecyl sulfate-polyacrylamide gel electrophoresis profile of purified WSSV virions by liquid chromatography-mass spectrometry (LC-MS/MS), we found that this giant polypeptide, designated VP664, is one of the viral structural proteins. The existence of the corresponding 18-kb transcript was confirmed by sequencing analysis of reverse transcription-PCR products, which also showed that vp664 was intron-less. A time course analysis showed that this transcript was actively transcribed at the late stage, suggesting that this gene product should contribute primarily to the assembly and morphogenesis of the virion. Several polyclonal antisera against this giant protein were prepared, and one of them was successfully used for immunoelectron microscopy analysis to localize the protein in the virion. Immunoelectron microscopy with a gold-labeled secondary antibody showed that the gold particles were regularly distributed around the periphery of the nucleocapsid with a periodicity that matched the characteristic stacked ring subunits that appear as striations. From this and other evidence, we argue that this giant ORF in fact encodes the major WSSV nucleocapsid protein.
Figures






Similar articles
-
Identification of the nucleocapsid, tegument, and envelope proteins of the shrimp white spot syndrome virus virion.J Virol. 2006 Mar;80(6):3021-9. doi: 10.1128/JVI.80.6.3021-3029.2006. J Virol. 2006. PMID: 16501111 Free PMC article.
-
Identification of two major virion protein genes of white spot syndrome virus of shrimp.Virology. 2000 Jan 20;266(2):227-36. doi: 10.1006/viro.1999.0088. Virology. 2000. PMID: 10639309
-
Characterization of a novel envelope protein WSV010 of shrimp white spot syndrome virus and its interaction with a major viral structural protein VP24.Virology. 2007 Jul 20;364(1):208-13. doi: 10.1016/j.virol.2007.02.030. Epub 2007 Apr 2. Virology. 2007. PMID: 17400271
-
Identification of an envelope protein (VP39) gene from shrimp white spot syndrome virus.Arch Virol. 2006 Jan;151(1):71-82. doi: 10.1007/s00705-005-0612-z. Epub 2005 Aug 23. Arch Virol. 2006. PMID: 16132182
-
Identification and characterization of a prawn white spot syndrome virus gene that encodes an envelope protein VP31.Virology. 2005 Sep 15;340(1):125-32. doi: 10.1016/j.virol.2005.06.007. Virology. 2005. PMID: 16023692
Cited by
-
Identification of the nucleocapsid, tegument, and envelope proteins of the shrimp white spot syndrome virus virion.J Virol. 2006 Mar;80(6):3021-9. doi: 10.1128/JVI.80.6.3021-3029.2006. J Virol. 2006. PMID: 16501111 Free PMC article.
-
Viral disease emergence in shrimp aquaculture: origins, impact and the effectiveness of health management strategies.Rev Aquac. 2009 Jun;1(2):125-154. doi: 10.1111/j.1753-5131.2009.01007.x. Epub 2009 May 15. Rev Aquac. 2009. PMID: 32328167 Free PMC article. Review.
-
A 3D model of the membrane protein complex formed by the white spot syndrome virus structural proteins.PLoS One. 2010 May 19;5(5):e10718. doi: 10.1371/journal.pone.0010718. PLoS One. 2010. PMID: 20502662 Free PMC article.
-
Antiviral therapy in shrimp through plant virus VLP containing VP28 dsRNA against WSSV.Beilstein J Org Chem. 2021 Jun 1;17:1360-1373. doi: 10.3762/bjoc.17.95. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34136015 Free PMC article.
-
Crustacean Genome Exploration Reveals the Evolutionary Origin of White Spot Syndrome Virus.J Virol. 2019 Jan 17;93(3):e01144-18. doi: 10.1128/JVI.01144-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404800 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Bang, M. L., T. Centner, F. Fornoff, A. J. Geach, M. Gotthardt, M. McNabb, C. C. Witt, D. Labeit, C. C. Gregorio, H. Granzier, and S. Labeit. 2001. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 89:1065-1072. - PubMed
-
- Baqui, M. M., N. De Moraes, R. V. Milder, and J. Pudles. 2000. A giant phosphoprotein localized at the spongiome region of Crithidia luciliae thermophila. J. Eukaryot. Microbiol. 47:532-537. - PubMed
-
- Baqui, M. M., C. S. Takata, R. V. Milder, and J. Pudles. 1996. A giant protein associated with the anterior pole of a trypanosomatid cell body skeleton. Eur. J. Cell Biol. 70:243-249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

