Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge
- PMID: 15596812
- PMCID: PMC538742
- DOI: 10.1128/JVI.79.1.159-175.2005
Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge
Abstract
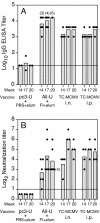
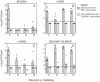
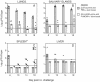
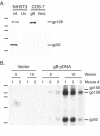
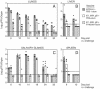
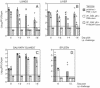
We previously demonstrated that vaccination of BALB/c mice with a pool of 13 plasmid DNAs (pDNAs) expressing murine cytomegalovirus (MCMV) genes followed by formalin-inactivated MCMV (FI-MCMV) resulted in complete protection against viral replication in the spleen and salivary glands following sublethal intraperitoneal (i.p.) challenge. Here, we found that following intranasal (i.n.) challenge, titers of virus in the lungs of the immunized mice were reduced approximately 1,000-fold relative to those for mock-immunized controls. We next sought to extend these results and to determine whether similar protection levels could be achieved by priming with a pool of three pDNAs containing three key plasmids (IE1, M84, and gB). We found that the three-pDNA priming elicited IE1- and M84-p65-specific CD8+ T lymphocytes and, following FI-MCMV boost, high levels of virion-specific immunoglobulin G (IgG) and virus-neutralizing antibodies. When mice were i.n. challenged 4 months after the last boost, titers of virus in the lungs of immunized mice were reduced 1,000- to 2,000-fold from those for controls during the peak of viral replication. Additionally, titers of virus were either at or below the detection limits for the salivary glands, liver, and spleen of the majority of the immunized mice. Following sublethal i.p. challenge, virus was undetectable in all of the above target organs of the immunized mice. Virion-specific IgA in the lungs was consistently detected by day 6 post-i.n. challenge for the immunized mice and by day 14 for controls. These results demonstrate the immunity and high levels of protection of the priming-boosting vaccination against both systemic and mucosal challenge.
Figures

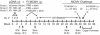

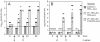
Similar articles
-
Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication.J Virol. 2002 May;76(10):4822-35. doi: 10.1128/jvi.76.10.4822-4835.2002. J Virol. 2002. PMID: 11967299 Free PMC article.
-
Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65).J Virol. 2000 Apr;74(8):3696-708. doi: 10.1128/jvi.74.8.3696-3708.2000. J Virol. 2000. PMID: 10729145 Free PMC article.
-
Strong CD8 T-cell responses following coimmunization with plasmids expressing the dominant pp89 and subdominant M84 antigens of murine cytomegalovirus correlate with long-term protection against subsequent viral challenge.J Virol. 2002 Mar;76(5):2100-12. doi: 10.1128/jvi.76.5.2100-2112.2002. J Virol. 2002. PMID: 11836387 Free PMC article.
-
Multiple epitopes in the murine cytomegalovirus early gene product M84 are efficiently presented in infected primary macrophages and contribute to strong CD8+-T-lymphocyte responses and protection following DNA immunization.J Virol. 2004 Oct;78(20):11233-45. doi: 10.1128/JVI.78.20.11233-11245.2004. J Virol. 2004. PMID: 15452242 Free PMC article.
-
An adjuvanted inactivated murine cytomegalovirus (MCMV) vaccine induces potent and long-term protective immunity against a lethal challenge with virulent MCMV.BMC Infect Dis. 2014 Apr 11;14:195. doi: 10.1186/1471-2334-14-195. BMC Infect Dis. 2014. PMID: 24720840 Free PMC article.
Cited by
-
Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus.PLoS One. 2008 Sep 22;3(9):e3256. doi: 10.1371/journal.pone.0003256. PLoS One. 2008. PMID: 18806877 Free PMC article.
-
Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease.J Virol. 2011 Apr;85(7):3461-72. doi: 10.1128/JVI.02521-10. Epub 2011 Jan 26. J Virol. 2011. PMID: 21270160 Free PMC article.
-
DNA vaccine prime followed by boost with live attenuated virus significantly improves antigen-specific T cell responses against human cytomegalovirus.Hum Vaccin Immunother. 2013 Oct;9(10):2120-32. doi: 10.4161/hv.25750. Epub 2013 Jul 25. Hum Vaccin Immunother. 2013. PMID: 24051429 Free PMC article.
-
Effective Immune Protection of Mice from Murine Cytomegalovirus Infection by Oral Salmonella-Based Vaccine Expressing Viral M78 Antigen.Vaccines (Basel). 2025 Jan 28;13(2):137. doi: 10.3390/vaccines13020137. Vaccines (Basel). 2025. PMID: 40006684 Free PMC article.
-
Developing a Vaccine against Congenital Cytomegalovirus (CMV) Infection: What Have We Learned from Animal Models? Where Should We Go Next?Future Virol. 2013 Dec;8(12):1161-1182. doi: 10.2217/fvl.13.106. Future Virol. 2013. PMID: 24523827 Free PMC article.
References
-
- Boeckh, M., W. G. Nichols, G. Papanicolaou, R. Rubin, J. R. Wingard, and J. Zaia. 2003. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol. Blood Marrow Transplant. 9:543-558. - PubMed
-
- Boppana, S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. - PubMed
-
- Boppana, S. B., L. B. Rivera, K. B. Fowler, M. Mach, and W. J. Britt. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366-1371. - PubMed
-
- Britt, W. J. 1996. Vaccines against human cytomegalovirus: time to test. Trends Microbiol. 4:34-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

