Silencing the morphogenesis of rotavirus
- PMID: 15596814
- PMCID: PMC538724
- DOI: 10.1128/JVI.79.1.184-192.2005
Silencing the morphogenesis of rotavirus
Abstract
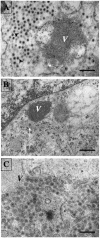
The morphogenesis of rotaviruses follows a unique pathway in which immature double-layered particles (DLPs) assembled in the cytoplasm bud across the membrane of the endoplasmic reticulum (ER), acquiring during this process a transient lipid membrane which is modified with the ER resident viral glycoproteins NSP4 and VP7; these enveloped particles also contain VP4. As the particles move towards the interior of the ER cisternae, the transient lipid membrane and the nonstructural protein NSP4 are lost, while the virus surface proteins VP4 and VP7 rearrange to form the outermost virus protein layer, yielding mature infectious triple-layered particles (TLPs). In this work, we have characterized the role of NSP4 and VP7 in rotavirus morphogenesis by silencing the expression of both glycoproteins through RNA interference. Silencing the expression of either NSP4 or VP7 reduced the yield of viral progeny by 75 to 80%, although the underlying mechanism of this reduction was different in each case. Blocking the synthesis of NSP4 affected the intracellular accumulation and the cellular distribution of several viral proteins, and little or no virus particles (neither DLPs nor TLPs) were assembled. VP7 silencing, in contrast, did not affect the expression or distribution of other viral proteins, but in its absence, enveloped particles accumulated within the lumen of the ER, and no mature infectious virus was produced. Altogether, these results indicate that during a viral infection, NSP4 serves as a receptor for DLPs on the ER membrane and drives the budding of these particles into the ER lumen, while VP7 is required for removing the lipid envelope during the final step of virus morphogenesis.
Figures



References
-
- Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

