Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection
- PMID: 15596816
- PMCID: PMC538712
- DOI: 10.1128/JVI.79.1.202-213.2005
Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection
Abstract
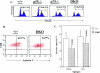
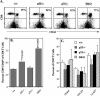
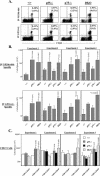
The role of tumor necrosis factor (TNF) in regulating various phases of the antiviral T-cell response is incompletely understood. Additionally, despite strong evidence ascribing a role for TNF in protecting against T-cell-dependent autoimmunity, the underlying mechanisms are still obscure. To address these issues, we have investigated the role of tumor necrosis factor receptors (TNFRs) I (p55R) and II (p75R) in regulating CD8 T-cell responses to lymphocytic choriomeningitis virus (LCMV) with wild-type, p55R-deficient (p55(-/-)), p75R-deficient (p75(-/-)), and p55R- and p75R-deficient (DKO) mice. Loss of p55R increased the number of memory CD8 T cells to only one of the two immunodominant epitopes, and p75R deficiency had a minimal impact on the T-cell response to LCMV. Strikingly, deficiency of both p55R and p75R had a more dramatic effect on the LCMV-specific CD8 T-cell response; in the DKO mice, as a sequel to enhanced expansion and a reduction in contraction of CD8 T cells, there was a substantial increase in the number of memory CD8 T cells (specific to the two immunodominant epitopes). While the majority of LCMV-specific memory CD8 T cells in wild-type mice were CD62Lhi CCR7hi (central memory), a major proportion of memory CD8 T cells in DKO mice were CD62Llo CCR7hi. TNFR deficiency did not affect the proliferative renewal of memory CD8 T cells. Taken together, these data suggested that TNFRs p55R and p75R have overlapping roles in downregulating CD8 T-cell responses and establishment of immune homeostasis during an acute viral infection.
Figures
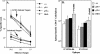



Similar articles
-
Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection.J Virol. 2004 Apr;78(8):3906-18. doi: 10.1128/jvi.78.8.3906-3918.2004. J Virol. 2004. PMID: 15047807 Free PMC article.
-
A role for TNF in limiting the duration of CTL effector phase and magnitude of CD8 T cell memory.J Leukoc Biol. 2007 Nov;82(5):1201-11. doi: 10.1189/jlb.0407240. Epub 2007 Aug 17. J Leukoc Biol. 2007. PMID: 17704295
-
Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection.J Virol. 2002 Jan;76(2):829-40. doi: 10.1128/jvi.76.2.829-840.2002. J Virol. 2002. PMID: 11752172 Free PMC article.
-
T cell responses to viral infections: lessons from lymphocytic choriomeningitis virus.Immunol Res. 2002;26(1-3):309-21. doi: 10.1385/IR:26:1-3:309. Immunol Res. 2002. PMID: 12403369 Review.
-
Induction and maintenance of CD8+ T cells specific for persistent viruses.Adv Exp Med Biol. 2007;590:121-37. doi: 10.1007/978-0-387-34814-8_9. Adv Exp Med Biol. 2007. PMID: 17191382 Review. No abstract available.
Cited by
-
Fecal microbiota transplantation from protozoa-exposed donors downregulates immune response in a germ-free mouse model, its role in immune response and physiology of the intestine.PLoS One. 2024 Oct 28;19(10):e0312775. doi: 10.1371/journal.pone.0312775. eCollection 2024. PLoS One. 2024. PMID: 39466773 Free PMC article.
-
TNF activity and T cells.Cytokine. 2018 Jan;101:14-18. doi: 10.1016/j.cyto.2016.08.003. Epub 2016 Aug 13. Cytokine. 2018. PMID: 27531077 Free PMC article. Review.
-
NFκB signaling in T cell memory.Front Immunol. 2023 Feb 24;14:1129191. doi: 10.3389/fimmu.2023.1129191. eCollection 2023. Front Immunol. 2023. PMID: 36911729 Free PMC article. Review.
-
TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice.J Clin Invest. 2007 Dec;117(12):3833-45. doi: 10.1172/JCI32567. J Clin Invest. 2007. PMID: 17992258 Free PMC article.
-
Targeting Regulatory T Cells by Addressing Tumor Necrosis Factor and Its Receptors in Allogeneic Hematopoietic Cell Transplantation and Cancer.Front Immunol. 2019 Aug 28;10:2040. doi: 10.3389/fimmu.2019.02040. eCollection 2019. Front Immunol. 2019. PMID: 31555271 Free PMC article. Review.
References
-
- Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. - PMC - PubMed
-
- Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. - PubMed
-
- Bachmann, R., H. P. Eugster, K. Frei, A. Fontana, and H. Lassmann. 1999. Impairment of TNF-receptor-1 signaling but not fas signaling diminishes T-cell apoptosis in myelin oligodendrocyte glycoprotein peptide-induced chronic demyelinating autoimmune encephalomyelitis in mice. Am. J. Pathol. 154:1417-1422. - PMC - PubMed
-
- Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T-cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

