Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters
- PMID: 15596843
- PMCID: PMC538722
- DOI: 10.1128/JVI.79.1.503-511.2005
Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters
Abstract
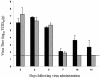
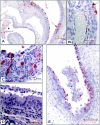
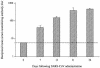
Small animal models are needed in order to evaluate the efficacy of candidate vaccines and antivirals directed against the severe acute respiratory syndrome coronavirus (SARS CoV). We investigated the ability of SARS CoV to infect 5-week-old Golden Syrian hamsters. When administered intranasally, SARS CoV replicates to high titers in the lungs and nasal turbinates. Peak replication in the lower respiratory tract was noted on day 2 postinfection (p.i.) and was cleared by day 7 p.i. Low levels of virus were present in the nasal turbinates of a few hamsters at 14 days p.i. Viral replication in epithelial cells of the respiratory tract was accompanied by cellular necrosis early in infection, followed by an inflammatory response coincident with viral clearance, focal consolidation in pulmonary tissue, and eventual pulmonary tissue repair. Despite high levels of virus replication and associated pathology in the respiratory tract, the hamsters showed no evidence of disease. Neutralizing antibodies were detected in sera at day 7 p.i., and mean titers at day 28 p.i. exceeded 1:400. Hamsters challenged with SARS CoV at day 28 p.i. were completely protected from virus replication and accompanying pathology in the respiratory tract. Comparing these data to the mouse model, SARS CoV replicates to a higher titer and for a longer duration in the respiratory tract of hamsters and is accompanied by significant pathology that is absent in mice. Viremia and extrapulmonary spread of SARS CoV to liver and spleen, which are seen in hamsters, were not detected in mice. The hamster, therefore, is superior to the mouse as a model for the evaluation of antiviral agents and candidate vaccines against SARS CoV replication.
Figures


References
-
- Bukreyev, A., E. W. Lamirande, U. J. Buchholz, L. N. Vogel, W. R. Elkins, M. St. Claire, B. R. Murphy, K. Subbarao, and P. L. Collins. 2004. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet 363:2122-2127. - PMC - PubMed
-
- Chong, P. Y., P. Chui, A. E. Ling, T. J. Franks, D. Y. Tai, Y. S. Leo, G. J. Kaw, G. Wansaicheong, K. P. Chan, L. L. Ean Oon, E. S. Teo, K. B. Tan, N. Nakajima, T. Sata, and W. D. Travis. 2004. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch. Pathol. Lab. Med. 128:195-204. - PubMed
-
- Donoghue, M., F. Hsieh, E. Baronas, K. Godbout, M. Gosselin, N. Stagliano, M. Donovan, B. Woolf, K. Robison, R. Jeyaseelan, R. E. Breitbart, and S. Acton. 2000. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circulation Res. 87:1-9. - PubMed
-
- Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

