Nonhepatic cell lines HeLa and 293 support efficient replication of the hepatitis C virus genotype 2a subgenomic replicon
- PMID: 15596851
- PMCID: PMC538706
- DOI: 10.1128/JVI.79.1.592-596.2005
Nonhepatic cell lines HeLa and 293 support efficient replication of the hepatitis C virus genotype 2a subgenomic replicon
Abstract
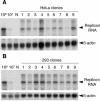
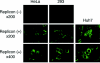
The hepatitis C virus (HCV) genotype 2a subgenomic replicon can replicate in two human non-hepatocyte-derived cell lines, HeLa and 293, with in vitro-transcribed replicon RNA. Sequencing analysis revealed that mutations in HCV-derived regions were not essential for replication in these cells, as some clones displayed no mutations.
Figures


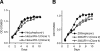
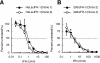
Similar articles
-
Replication of hepatitis C virus genotype 3a in cultured cells.Gastroenterology. 2013 Jan;144(1):56-58.e7. doi: 10.1053/j.gastro.2012.09.017. Epub 2012 Sep 19. Gastroenterology. 2013. PMID: 22999961
-
Genotype 2a hepatitis C virus subgenomic replicon can replicate in HepG2 and IMY-N9 cells.J Biol Chem. 2004 May 21;279(21):22371-6. doi: 10.1074/jbc.M311120200. Epub 2004 Feb 26. J Biol Chem. 2004. PMID: 14990575
-
[Replication of hepatitis C virus genome].Uirusu. 2008 Dec;58(2):191-8. Uirusu. 2008. PMID: 19374197 Review. Japanese.
-
cDNA microarray analysis to compare HCV subgenomic replicon cells with their cured cells.Virus Res. 2005 Jan;107(1):73-81. doi: 10.1016/j.virusres.2004.06.013. Virus Res. 2005. PMID: 15567036
-
[Establishment of cell lines expressing HCV replicon and its applications].Nihon Rinsho. 2004 Jul;62 Suppl 7(Pt 1):116-20. Nihon Rinsho. 2004. PMID: 15359775 Review. Japanese. No abstract available.
Cited by
-
Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma.Drug Deliv. 2017 Nov;24(1):20-29. doi: 10.1080/10717544.2016.1225856. Drug Deliv. 2017. PMID: 28155331 Free PMC article.
-
Replication of a hepatitis C virus replicon clone in mouse cells.Virol J. 2006 Oct 28;3:89. doi: 10.1186/1743-422X-3-89. Virol J. 2006. PMID: 17069661 Free PMC article.
-
Two methods of heterokaryon formation to discover HCV restriction factors.J Vis Exp. 2012 Jul 16;(65):e4029. doi: 10.3791/4029. J Vis Exp. 2012. PMID: 22825033 Free PMC article.
-
EWSR1 binds the hepatitis C virus cis-acting replication element and is required for efficient viral replication.J Virol. 2013 Jun;87(12):6625-34. doi: 10.1128/JVI.01006-12. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552423 Free PMC article.
-
The Serum Very-Low-Density Lipoprotein Serves as a Restriction Factor against Hepatitis C Virus Infection.J Virol. 2015 Jul;89(13):6782-91. doi: 10.1128/JVI.00194-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903344 Free PMC article.
References
-
- Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. - PubMed
-
- Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. - PubMed
-
- Date, T., T. Kato, M. Miyamoto, Z. Zhao, K. Yasui, M. Mizokami, and T. Wakita. 2004. Genotype 2a hepatitis C virus subgenomic replicon can replicate in HepG2 and IMY-N9 cells. J. Biol. Chem. 279:22371-22376. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

