Synchronized infection of cell cultures by magnetically controlled virus
- PMID: 15596857
- PMCID: PMC538731
- DOI: 10.1128/JVI.79.1.622-625.2005
Synchronized infection of cell cultures by magnetically controlled virus
Abstract
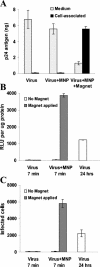
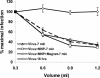
To override the diffusion-limited adsorption step of viral infection, we magnetically synchronized cell attachment. Human immunodeficiency virus type 1-based lentivirus preparations were rendered magnetically reactive by association with magnetite nanoparticles, 50 nm in diameter. Application of a magnetic field resulted in immediate redistribution of the viral inoculum to the cell-associated state and completion of the productive adsorption process within 1 min. Independent of adsorption time, viral concentration, and diffusion rate, infection subsequently progressed by the receptor-mediated entry mechanism. Synchronization of this rate-limiting step of infection may now be applied to analyze isolated events in the viral replication sequence.
Figures
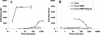
Similar articles
-
Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2.EMBO J. 2007 Sep 5;26(17):4016-28. doi: 10.1038/sj.emboj.7601831. Epub 2007 Aug 23. EMBO J. 2007. PMID: 17717529 Free PMC article.
-
Efficiency of porcine endothelial cell infection with human cytomegalovirus depends on both virus tropism and endothelial cell vascular origin.Xenotransplantation. 2010 Jul-Aug;17(4):274-87. doi: 10.1111/j.1399-3089.2010.00594.x. Xenotransplantation. 2010. PMID: 20723200
-
Dengue virus type 2 infects human endothelial cells through binding of the viral envelope glycoprotein to cell surface polypeptides.J Gen Virol. 2003 Nov;84(Pt 11):3095-3098. doi: 10.1099/vir.0.19308-0. J Gen Virol. 2003. PMID: 14573814
-
The role of herpes simplex virus glycoproteins in the virus replication cycle.Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
-
[Envelope and membrane glycoproteins of Herpes simplex virus].Rev Latinoam Microbiol. 1992 Jan-Mar;34(1):23-31. Rev Latinoam Microbiol. 1992. PMID: 1345300 Review. Spanish.
Cited by
-
Genetic Engineering of Primary Mouse Intestinal Organoids Using Magnetic Nanoparticle Transduction Viral Vectors for Frozen Sectioning.J Vis Exp. 2019 May 10;(147):10.3791/57040. doi: 10.3791/57040. J Vis Exp. 2019. PMID: 31132065 Free PMC article.
-
Optimized Infectivity of the Cell-Free Single-Cycle Human Immunodeficiency Viruses Type 1 (HIV-1) and Its Restriction by Host Cells.PLoS One. 2013 Jun 18;8(6):e67170. doi: 10.1371/journal.pone.0067170. Print 2013. PLoS One. 2013. PMID: 23825637 Free PMC article.
-
Identification and characterization of persistent intracellular human immunodeficiency virus type 1 integrase strand transfer inhibitor activity.Antimicrob Agents Chemother. 2011 Jan;55(1):42-9. doi: 10.1128/AAC.01064-10. Epub 2010 Nov 8. Antimicrob Agents Chemother. 2011. PMID: 21060108 Free PMC article.
-
Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies.PLoS Pathog. 2012;8(4):e1002634. doi: 10.1371/journal.ppat.1002634. Epub 2012 Apr 5. PLoS Pathog. 2012. PMID: 22496655 Free PMC article. Clinical Trial.
-
Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds.J Virol. 2017 Jan 18;91(3):e01880-16. doi: 10.1128/JVI.01880-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881646 Free PMC article.
References
-
- Alian, A., A. Eldor, H. Falk, and A. Panet. 2002. Viral mediated gene transfer to sprouting blood vessels during angiogenesis. J. Virol. Methods 105:1-11. - PubMed
-
- Allison, A. C., and R. C. Valentine. 1960. Viral particle adsorption. III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim. Biophys. Acta 40:400-410. - PubMed
-
- Axelrod, J. H., and A. Honigman. 1999. A sensitive and versatile bioluminescence bioassay for HIV type 1 based on adenoviral vectors. AIDS Res. Hum. Retrovir. 15:759-767. - PubMed
-
- Blumenzweig, I., L. Baraz, A. Friedler, U. H. Danielson, C. Gilon, M. Steinitz, and M. Kotler. 2002. HIV-1 Vif-derived peptide inhibits drug-resistant HIV proteases. Biochem. Biophys. Res. Commun. 292:832-840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

