Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L
- PMID: 15598747
- PMCID: PMC535703
- DOI: 10.1073/pnas.0405971102
Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L
Abstract
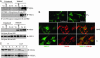
Neurofibromatosis 2 (NF2) is a tumor suppressor, although the molecular mechanism accounting for this effect remains unknown. Here, we show that merlin exerts its activity by inhibiting phosphatidylinositol 3-kinase (PI3-kinase), through binding to PIKE-L. Wild-type merlin, but not patient-derived mutant (L64P), binds PIKE-L and inhibits PI3-kinase activity. This suppression of PI3-kinase activity results from merlin disrupting the binding of PIKE-L to PI3-kinase. In addition, merlin suppression of PI3-kinase activity as well as schwannoma cell growth is abrogated by a single PIKE-L point mutation (P187L) that cannot bind merlin but can still activate PI3-kinase. Knocking down PIKE-L with RNA interference abolishes merlin's tumor-suppressive activity. Our data support the hypothesis that PIKE-L is an important mediator of merlin growth suppression.
Figures


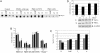
References
-
- Eldridge, R. (1981) Adv. Neurol. 29, 57–65. - PubMed
-
- Rouleau, G. A., Merel, P., Lutchman, M., Sanson, M., Zucman, J., Marineau, C., Hoang-Xuan, K., Demczuk, S., Desmaze, C., Plougastel, B., et al. (1993) Nature 363, 515–521. - PubMed
-
- Trofatter, J. A., MacCollin, M. M., Rutter, J. L., Murrell, J. R., Duyao, M. P., Parry, D. M., Eldridge, R., Kley, N., Menon, A. G., Pulaski, K., et al. (1993) Cell 72, 791–800. - PubMed
-
- Gutmann, D. H. (1997) Neurobiol. Dis. 3, 247–261. - PubMed
-
- Gonzalez-Agosti, C., Xu, L., Pinney, D., Beauchamp, R., Hobbs, W., Gusella, J. & Ramesh, V. (1996) Oncogene 13, 1239–1247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

