The Rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin
- PMID: 15598797
- PMCID: PMC544493
- DOI: 10.1105/tpc.104.028357
The Rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin
Abstract
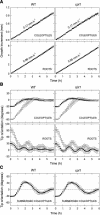
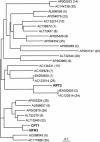
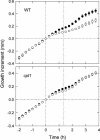
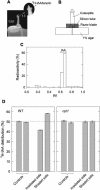
We isolated a mutant, named coleoptile phototropism1 (cpt1), from gamma-ray-mutagenized japonica-type rice (Oryza sativa). This mutant showed no coleoptile phototropism and severely reduced root phototropism after continuous stimulation. A map-based cloning strategy and transgenic complementation test were applied to demonstrate that a NPH3-like gene deleted in the mutant corresponds to CPT1. Phylogenetic analysis of putative CPT1 homologs of rice and related proteins indicated that CPT1 has an orthologous relationship with Arabidopsis thaliana NPH3. These results, along with those for Arabidopsis, demonstrate that NPH3/CPT1 is a key signal transduction component of higher plant phototropism. In an extended study with the cpt1 mutant, it was found that phototropic differential growth is accompanied by a CPT1-independent inhibition of net growth. Kinetic investigation further indicated that a small phototropism occurs in cpt1 coleoptiles. This response, induced only transiently, was thought to be caused by the CPT1-independent growth inhibition. The 3H-indole-3-acetic acid applied to the coleoptile tip was asymmetrically distributed between the two sides of phototropically responding coleoptiles. However, no asymmetry was induced in cpt1 coleoptiles, indicating that lateral translocation of auxin occurs downstream of CPT1. It is concluded that the CPT1-dependent major phototropism of coleoptiles is achieved by lateral auxin translocation and subsequent growth redistribution.
Figures




Similar articles
-
NPH3- and PGP-like genes are exclusively expressed in the apical tip region essential for blue-light perception and lateral auxin transport in maize coleoptiles.J Exp Bot. 2011 Jun;62(10):3459-66. doi: 10.1093/jxb/err019. Epub 2011 Mar 31. J Exp Bot. 2011. PMID: 21459767 Free PMC article.
-
The gene MACCHI-BOU 4/ENHANCER OF PINOID encodes a NPH3-like protein and reveals similarities between organogenesis and phototropism at the molecular level.Development. 2007 Nov;134(21):3849-59. doi: 10.1242/dev.009654. Epub 2007 Oct 3. Development. 2007. PMID: 17913786
-
The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism.Plant Cell. 2012 Feb;24(2):551-65. doi: 10.1105/tpc.111.094284. Epub 2012 Feb 28. Plant Cell. 2012. PMID: 22374399 Free PMC article.
-
Molecular genetic analysis of phototropism in Arabidopsis.Plant Cell Physiol. 2012 Sep;53(9):1517-34. doi: 10.1093/pcp/pcs111. Epub 2012 Aug 3. Plant Cell Physiol. 2012. PMID: 22864452 Free PMC article. Review.
-
Phototropism involves a lateral gradient of growth inhibitors, not of auxin. A review.Environ Exp Bot. 1989 Jan;29(1):25-36. doi: 10.1016/0098-8472(89)90036-1. Environ Exp Bot. 1989. PMID: 11541033 Review.
Cited by
-
Blue light does not inhibit nodulation in Sesbania rostrata.Plant Signal Behav. 2017 Jan 2;12(1):e1268313. doi: 10.1080/15592324.2016.1268313. Plant Signal Behav. 2017. PMID: 27935414 Free PMC article.
-
Elucidating the genetic basis of biomass accumulation and radiation use efficiency in spring wheat and its role in yield potential.Plant Biotechnol J. 2019 Jul;17(7):1276-1288. doi: 10.1111/pbi.13052. Epub 2019 Jan 15. Plant Biotechnol J. 2019. PMID: 30549213 Free PMC article.
-
Functional Analysis of MAX2 in Phototropins-Mediated Cotyledon Flattening in Arabidopsis.Front Plant Sci. 2018 Oct 17;9:1507. doi: 10.3389/fpls.2018.01507. eCollection 2018. Front Plant Sci. 2018. PMID: 30386362 Free PMC article.
-
Predicting the size of the progeny mapping population required to positionally clone a gene.Genetics. 2007 Aug;176(4):2035-54. doi: 10.1534/genetics.107.074377. Epub 2007 Jun 11. Genetics. 2007. PMID: 17565938 Free PMC article.
-
Possible involvement of phototropins in leaf movement of kidney bean in response to blue light.Plant Physiol. 2005 Aug;138(4):1994-2004. doi: 10.1104/pp.105.062026. Epub 2005 Jul 22. Plant Physiol. 2005. PMID: 16040656 Free PMC article.
References
-
- Baba, T., et al. (2000). Construction and characterization of rice genomic libraries, PAC library of japonica variety Nipponbare, and BAC library of indica variety Kasalath. Bull. Natl. Inst. Agrobiol. Resour. 14, 41–49.
-
- Biswas, K.K., and Iino, M. (2002). A mutant of rice impaired specifically in the phototropism of coleoptiles. J. Plant Res. 115 (suppl.), 71. - PubMed
-
- Biswas, K.K., Neumann, R., Haga, K., Yatoh, O., and Iino, M. (2003). Photomorphogenesis of rice seedlings: A mutant impaired in phytochrome-mediated inhibition of coleoptile growth. Plant Cell Physiol. 44, 242–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

