Metabolic sensitivity of pancreatic tumour cell apoptosis to glycogen phosphorylase inhibitor treatment
- PMID: 15599384
- PMCID: PMC2409791
- DOI: 10.1038/sj.bjc.6602243
Metabolic sensitivity of pancreatic tumour cell apoptosis to glycogen phosphorylase inhibitor treatment
Abstract
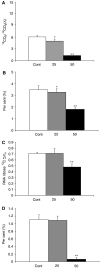
Inhibitors of glycogen breakdown regulate glucose homeostasis by limiting glucose production in diabetes. Here we demonstrate that restrained glycogen breakdown also inhibits cancer cell proliferation and induces apoptosis through limiting glucose oxidation, as well as nucleic acid and de novo fatty acid synthesis. Increasing doses (50-100 microM) of the glycogen phosphorylase inhibitor CP-320626 inhibited [1,2-(13)C(2)]glucose stable isotope substrate re-distribution among glycolysis, pentose and de novo fatty acid synthesis in MIA pancreatic adenocarcinoma cells. Limited oxidative pentose-phosphate synthesis, glucose contribution to acetyl CoA and de novo fatty acid synthesis closely correlated with decreased cell proliferation. The stable isotope-based dynamic metabolic profile of MIA cells indicated a significant dose-dependent decrease in macromolecule synthesis, which was detected at lower drug doses and before the appearance of apoptosis markers. Normal fibroblasts (CRL-1501) did not show morphological or metabolic signs of apoptosis likely due to their slow rate of growth and metabolic activity. This indicates that limiting carbon re-cycling and rapid substrate mobilisation from glycogen may be an effective and selective target site for new drug development in rapidly dividing cancer cells. In conclusion, pancreatic cancer cell growth arrest and death are closely associated with a characteristic decrease in glycogen breakdown and glucose carbon re-distribution towards RNA/DNA and fatty acids during CP-320626 treatment.
Figures



Similar articles
-
Inhibition of glycogen phosphorylation induces changes in cellular proteome and signaling pathways in MIA pancreatic cancer cells.Pancreas. 2012 Apr;41(3):397-408. doi: 10.1097/MPA.0b013e318236f022. Pancreas. 2012. PMID: 22158071 Free PMC article.
-
Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice.Gastroenterology. 2017 Jul;153(1):277-291.e19. doi: 10.1053/j.gastro.2017.03.008. Epub 2017 Mar 15. Gastroenterology. 2017. PMID: 28315323
-
Inhibition of glycogen phosphorylase (GP) by CP-91,149 induces growth inhibition correlating with brain GP expression.Biochem Biophys Res Commun. 2003 Sep 12;309(1):126-34. doi: 10.1016/s0006-291x(03)01542-0. Biochem Biophys Res Commun. 2003. PMID: 12943673
-
Physiological control of liver glycogen metabolism: lessons from novel glycogen phosphorylase inhibitors.Mini Rev Med Chem. 2010 Oct;10(12):1175-87. doi: 10.2174/1389557511009011175. Mini Rev Med Chem. 2010. PMID: 20716056 Review.
-
Metabolic biomarker and kinase drug target discovery in cancer using stable isotope-based dynamic metabolic profiling (SIDMAP).Curr Cancer Drug Targets. 2003 Dec;3(6):445-53. doi: 10.2174/1568009033481769. Curr Cancer Drug Targets. 2003. PMID: 14683502 Review.
Cited by
-
The Warburg effect: a balance of flux analysis.Metabolomics. 2015 Aug;11(4):787-796. doi: 10.1007/s11306-014-0760-9. Metabolomics. 2015. PMID: 26207106 Free PMC article.
-
Variations in Glycogen Synthesis in Human Pluripotent Stem Cells with Altered Pluripotent States.PLoS One. 2015 Nov 13;10(11):e0142554. doi: 10.1371/journal.pone.0142554. eCollection 2015. PLoS One. 2015. PMID: 26565809 Free PMC article.
-
Glycogen phosphorylase inhibition improves beta cell function.Br J Pharmacol. 2018 Jan;175(2):301-319. doi: 10.1111/bph.13819. Epub 2017 Jun 18. Br J Pharmacol. 2018. PMID: 28409826 Free PMC article.
-
Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma.Cell Death Dis. 2016 May 5;7(5):e2213. doi: 10.1038/cddis.2016.117. Cell Death Dis. 2016. PMID: 27148686 Free PMC article.
-
Metabolic Consequences of LDHA inhibition by Epigallocatechin Gallate and Oxamate in MIA PaCa-2 Pancreatic Cancer Cells.Metabolomics. 2015 Feb;11(1):71-80. doi: 10.1007/s11306-014-0672-8. Metabolomics. 2015. PMID: 26246802 Free PMC article.
References
-
- Boren J, Cascante M, Marin S, Comin-Anduix B, Centelles JJ, Lim S, Bassilian S, Ahmed S, Lee WN, Boros LG (2001) Gleevec (STI571) influences metabolic enzyme activities and glucose carbon flow toward nucleic acid and fatty acid synthesis in myeloid tumor cells. J Biol Chem 276: 37747–37753 - PubMed
-
- Boros LG, Cascante M, Lee WN (2002a) Metabolic profiling of cell growth and death in cancer: applications in drug discovery. Drug Discov Today 7: 364–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

