Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer
- PMID: 15599396
- PMCID: PMC535068
- DOI: 10.1172/JCI22123
Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer
Abstract
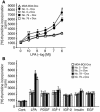
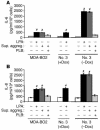
The role of lysophosphatidic acid (LPA) in cancer is poorly understood. Here we provide evidence for a role of LPA in the progression of breast cancer bone metastases. LPA receptors LPA(1), LPA(2), and LPA(3) were expressed in human primary breast tumors and a series of human breast cancer cell lines. The inducible overexpression of LPA(1) in MDA-BO2 breast cancer cells specifically sensitized these cells to the mitogenic action of LPA in vitro. In vivo, LPA(1) overexpression in MDA-BO2 cells enhanced the growth of subcutaneous tumor xenografts and promoted bone metastasis formation in mice by increasing both skeletal tumor growth and bone destruction. This suggested that endogenous LPA was produced in the tumor microenvironment. However, MDA-BO2 cells or transfectants did not produce LPA. Instead, they induced the release of LPA from activated platelets which, in turn, promoted tumor cell proliferation and the LPA(1)-dependent secretion of IL-6 and IL-8, 2 potent bone resorption stimulators. Moreover, platelet-derived LPA deprivation in mice, achieved by treatment with the platelet antagonist Integrilin, inhibited the progression of bone metastases caused by parental and LPA(1)-overexpressing MDA-BO2 cells and reduced the progression of osteolytic lesions in mice bearing CHO-beta3wt ovarian cancer cells. Overall, our data suggest that, at the bone metastatic site, tumor cells stimulate the production of LPA from activated platelets, which enhances both tumor growth and cytokine-mediated bone destruction.
Figures









Comment in
-
Platelets and metastasis revisited: a novel fatty link.J Clin Invest. 2004 Dec;114(12):1691-3. doi: 10.1172/JCI23823. J Clin Invest. 2004. PMID: 15599391 Free PMC article. Review.
References
-
- An S, Dickens MA, Bleu T, Hallmark OG, Goetzl EJ. Molecular cloning of the human Edg2 protein and its identification as a functional cellular receptor for lysophosphatidic acid. Biochem. Biophys. Res. Commun. 1997;231:619–622. - PubMed
-
- An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 1998;273:7906–7910. - PubMed
-
- Bandoh K, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 1999;274:27776–27785. - PubMed
-
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a Novel G Protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 2003;278:25600–25606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

