Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes
- PMID: 15599402
- PMCID: PMC535069
- DOI: 10.1172/JCI22284
Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes
Abstract
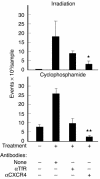
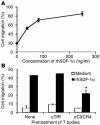
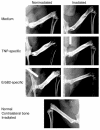
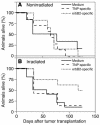
Prostate cancer is currently the most commonly diagnosed noncutaneous malignancy in American men. When metastatic, usually to the bone, the disease is no longer curable and is usually treated palliatively with androgen ablation. However, after conversion to androgen-independent disease, there is no effective therapy currently available. The "T body" approach, which uses genetically reprogrammed lymphocytes derived from the patient and expressing chimeric receptor genes, combines the effector functions of T lymphocytes and NK cells with the ability of antibodies to recognize predefined surface antigens with high specificity and in a non-MHC-restricted manner. We show here the therapeutic efficacy of human lymphocytes bearing erbB2-specific chimeric receptors on human prostate cancer BM lesions in a SCID mouse model after conditioning of the recipient to allow homing and persistent functioning of the adoptively transferred cells. Induction of stromal cell-derived factor-1 production within the BM using low-dose irradiation or cyclophosphamide combined with IL-2 administration enhanced the homing of systemically delivered T bodies, resulting in decreased tumor growth and prostate-specific antigen secretion, prolongation of survival, and even cure of the treated mice. These preclinical studies strongly support the idea that the T body approach has therapeutic potential in disseminated prostate cancer.
Figures





References
-
- Jemal A, et al. Cancer statistics, 2003. CA Cancer J. Clin. 2003;53:5–26. - PubMed
-
- Yonou H, et al. Establishment of a novel species- and tissue-specific metastasis model of human prostate cancer in humanized non-obese diabetic/severe combined immunodeficient mice engrafted with human adult lung and bone. Cancer Res. 2001;61:2177–2182. - PubMed
-
- Whitmore WF., Jr Natural history and staging of prostate cancer. Urol. Clin. North. Am. 1984;11:205–220. - PubMed
-
- Hossain, N.H., and Crawford, D. 1997. Androgen deprivation strategies for metastatic prostate cancer. In Principles and practices of genitourniary oncology. D. Raghavan, H.I. Scher, S.A. Leibel, and P. Lange, editors. Lippincott—Raven. Philadelphia, Pennsylvania, USA. 591–597.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

