Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy
- PMID: 15599732
- PMCID: PMC11032914
- DOI: 10.1007/s00262-004-0593-x
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy
Abstract
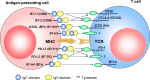
Programmed death receptor ligand 1 (PD-L1, also called B7-H1) is a recently described B7 family member. In contrast to B7-1 and B7-2, PD-L1 does not interact with either CD28 or CTLA-4. To date, one specific receptor has been identified that can be ligated by PD-L1. This receptor, programmed death receptor 1 (PD-1), has been shown to negatively regulate T-cell receptor (TCR) signaling. Upon ligating its receptor, PD-L1 has been reported to decrease TCR-mediated proliferation and cytokine production. PD-1 gene-deficient mice developed autoimmune diseases, which early led to the hypothesis of PD-L1 regulating peripheral tolerance. In contrast to normal tissues, which show minimal surface expression of PD-L1 protein, PD-L1 expression was found to be abundant on many murine and human cancers and could be further up-regulated upon IFN-gamma stimulation. Thus, PD-L1 might play an important role in tumor immune evasion. This review discusses the currently available data concerning negative T-cell regulation via PD-1, the blockade of PD-L1/PD-1 interactions, and the implications for adoptive T-cell therapies.
Figures

References
-
- Abbott A. Cancer research: on the offensive. Nature. 2002;416:470–474. - PubMed
-
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. - PubMed
-
- Bachmann MF, Waterhouse P, Speiser DE, McKall-Faienza K, Mak TW, Ohashi PS. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J Immunol. 1998;160:95–100. - PubMed
-
- Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

