Intraoperative infrared imaging of brain tumors
- PMID: 15599965
- PMCID: PMC4294194
- DOI: 10.3171/jns.2004.101.6.0960
Intraoperative infrared imaging of brain tumors
Abstract
Object: Although clinical imaging defines the anatomical relationship between a brain tumor and the surrounding brain and neurological deficits indicate the neurophysiological consequences of the tumor, the effect of a brain tumor on vascular physiology is less clear.
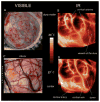
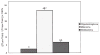
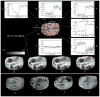
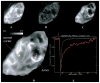
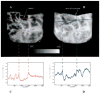
Methods: An infrared camera was used to measure the temperature of the cortical surface before, during, and after removal of a mass in 34 patients (primary brain tumor in 21 patients, brain metastases in 10 and falx meningioma, cavernous angioma, and radiation necrosis-astrocytosis in one patient each). To establish the magnitude of the effect on blood flow induced by the tumor, the images were compared with those from a group of six patients who underwent temporal lobectomy for epilepsy. In four cases a cerebral artery was temporarily occluded during the course of the surgery and infrared emissions from the cortex before and after occlusion were compared to establish the relationship of local temperature to regional blood flow. Discrete temperature gradients were associated with surgically verified lesions in all cases. Depending on the type of tumor, the cortex overlying the tumor was either colder or warmer than the surrounding cortex. Spatial reorganization of thermal gradients was observed after tumor resection. Temperature gradients of the cortex in patients with tumors exceeded those measured in the cortex of patients who underwent epilepsy surgery.
Conclusions: Brain tumors induce changes in cerebral blood flow (CBF) in the cortex, which can be made visible by performing infrared imaging during cranial surgery. A reduction in CBF beyond the tumor margin improves after removal of the lesion.
Figures

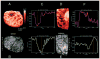
References
-
- Anbar M, Brown C, Milescu L, et al. The potential of dynamic area telethermometry in assessing breast cancer. IEEE Eng Med Biol Mag. 2000;19:58–62. - PubMed
-
- Behrens PF, Ostertag CB, Warnke PC. Regional cerebral blood flow in peritumoral brain edema during dexamethasone treatment: a xenon-enhanced computed tomographic study. Neurosurgery. 1998;43:235–241. - PubMed
-
- Capon A, Hildebrand J, Verbist A, et al. Changes in regional cerebral blood flows produced by dexamethasone in patients with brain metastases. Acta Neurol Belg. 1976;76:325–330. - PubMed
-
- Di Chiro G, DeLaPaz RL, Brooks RA, et al. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology. 1982;32:1323–1329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

