Comparing estimates of cost effectiveness submitted to the National Institute for Clinical Excellence (NICE) by different organisations: retrospective study
- PMID: 15601681
- PMCID: PMC543863
- DOI: 10.1136/bmj.38285.482350.82
Comparing estimates of cost effectiveness submitted to the National Institute for Clinical Excellence (NICE) by different organisations: retrospective study
Abstract
Objective: To assess the association between different types of organisation and the results from economic evaluations.
Design: Retrospective pairwise comparison of evidence submitted to the technology appraisal programme of the National Institute for Clinical Excellence (NICE) by manufacturers of the relevant healthcare technologies and by contracted university based assessment groups.
Data sources: Data from the first 62 appraisals.
Main outcome measure: Incremental cost effectiveness ratios.
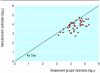
Results: Data from 27 of the 62 appraisals could be compared. The analysis of 54 pairwise comparisons showed that manufacturers' estimates of incremental cost effectiveness ratios were lower (suggesting a more cost effective use of resources) than those produced by the assessment groups (25 were lower, 29 were the same, none were higher, P < 0.01). Restriction of this dataset to include only one pairwise comparison per appraisal (27 pairs) produced a similar result (21 were lower, two were the same, four were higher, P < 0.001).
Conclusions: The estimated incremental cost effectiveness ratios submitted by manufacturers were on average significantly lower than those submitted by the assessment groups. These results show that an important role of NICE's appraisal committee, and of decision makers in general, is to determine which economic evaluations, or parts of evaluations, should be given more credence.
Figures

References
-
- Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA 2003;290: 921-8. - PubMed
-
- Friedberg M, Saffran B, Stinson TJ, Nelson W, Bennett CL. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. JAMA 1999;15: 1453-7. - PubMed
-
- Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press, 1997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
