Molecular analysis of the Vibrio cholerae type II secretion ATPase EpsE
- PMID: 15601709
- PMCID: PMC538811
- DOI: 10.1128/JB.187.1.249-256.2005
Molecular analysis of the Vibrio cholerae type II secretion ATPase EpsE
Abstract
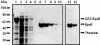
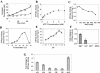
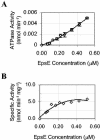
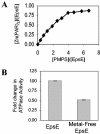
The type II secretion system is a macromolecular assembly that facilitates the extracellular translocation of folded proteins in gram-negative bacteria. EpsE, a member of this secretion system in Vibrio cholerae, contains a nucleotide-binding motif composed of Walker A and B boxes that are thought to participate in binding and hydrolysis of ATP and displays structural homology to other transport ATPases. Here we demonstrate that purified EpsE is an Mg2+-dependent ATPase and define optimal conditions for the hydrolysis reaction. EpsE displays concentration-dependent activity, which may suggest that the active form is oligomeric. Size exclusion chromatography showed that the majority of purified EpsE is monomeric; however, detailed analyses of specific activities obtained following gel filtration revealed the presence of a small population of active oligomers. We further report that EpsE binds zinc through a tetracysteine motif near its carboxyl terminus, yet metal displacement assays suggest that zinc is not required for catalysis. Previous studies describing interactions between EpsE and other components of the type II secretion pathway together with these data further support the hypothesis that EpsE functions to couple energy to the type II apparatus, thus enabling secretion.
Figures


References
-
- Banecki, B., A. Wawrzynow, J. Puzewicz, C. Georgopoulos, and M. Zylicz. 2001. Structure-function analysis of the zinc-binding region of the Clpx molecular chaperone. J. Biol. Chem. 276:18843-18848. - PubMed
-
- Collet, J. F., J. C. D'Souza, U. Jakob, and J. C. Bardwell. 2003. Thioredoxin 2, an oxidative stress-induced protein, contains a high affinity zinc binding site. J. Biol. Chem. 278:45325-45332. - PubMed
-
- Donaldson, L. W., U. Wojtyra, and W. A. Houry. 2003. Solution structure of the dimeric zinc binding domain of the chaperone ClpX. J. Biol. Chem. 278:48991-48996. - PubMed
-
- Fekkes, P., J. G. de Wit, A. Boorsma, R. H. Friesen, and A. J. Driessen. 1999. Zinc stabilizes the SecB binding site of SecA. Biochemistry 38:5111-5116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

