Influenza virus entry and infection require host cell N-linked glycoprotein
- PMID: 15601777
- PMCID: PMC535801
- DOI: 10.1073/pnas.0405172102
Influenza virus entry and infection require host cell N-linked glycoprotein
Abstract
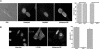
A widely held view of influenza virus infection is that the viral receptor consists of cell surface carbohydrate sialic acid, which can be present as glycoprotein or glycolipid. Here, we examined influenza virus entry and infection in Lec1 cells, a mutant CHO cell line deficient in terminal N-linked glycosylation caused by a mutation in the N-acetylglucosaminyltransferase I (GnT1) gene. We show that influenza virus cannot infect Lec1 cells, despite having full capacity to undergo virus binding and fusion. Lec1 cells also show no virus replication defect, and infection was restored in Lec1 cells expressing wild-type GnT1. Viruses were apparently arrested at the level of internalization from the plasma membrane and were not endocytosed. Lec1 cells were refractory to infection by several strains of influenza virus, including H1 and H3 strains of influenza A, as well as influenza B virus. Finally, cleavage of N-glycans from wild-type CHO cells markedly reduced infection by influenza virus. We suggest that influenza virus specifically requires N-linked glycoprotein for entry into cells, and that sialic acid, although acting as an efficient attachment factor, is not sufficient as an influenza virus receptor in vivo.
Figures

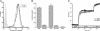


References
-
- Cox, N. J. & Subbarao, K. (2000) Annu. Rev. Med. 51, 407–421. - PubMed
-
- Skehel, J. J. & Wiley, D. C. (2000) Annu. Rev. Biochem. 69, 531–569. - PubMed
-
- Gottschalk, A. (1959) in The Viruses: Biochemical Biological and Biophysical Properties, eds. Burnet, F. M. & Stanley, W. M. (Academic, New York), Vol. 3, pp. 51–61.
-
- Steinhauer, D. A. & Wharton, S. A. (1998) in Textbook of Influenza, eds. Nicholson, K. G., Webster, R. G. & Hay, A. J. (Blackwell Science, Oxford), pp. 54–64.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

