In vivo analysis of growth hormone receptor signaling domains and their associated transcripts
- PMID: 15601831
- PMCID: PMC538772
- DOI: 10.1128/MCB.25.1.66-77.2005
In vivo analysis of growth hormone receptor signaling domains and their associated transcripts
Erratum in
- Mol Cell Biol. 2005 Mar;25(5):2072
Abstract
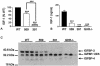
The growth hormone receptor (GHR) is a critical regulator of postnatal growth and metabolism. However, the GHR signaling domains and pathways that regulate these processes in vivo are not defined. We report the first knock-in mouse models with deletions of specific domains of the receptor that are required for its in vivo actions. Mice expressing truncations at residue m569 (plus Y539/545-F) and at residue m391 displayed a progressive impairment of postnatal growth with receptor truncation. Moreover, after 4 months of age, marked male obesity was observed in both mutant 569 and mutant 391 and was associated with hyperglycemia. Both mutants activated hepatic JAK2 and ERK2, whereas STAT5 phosphorylation was substantially decreased for mutant 569 and absent from mutant 391, correlating with loss of IGF-1 expression and reduction in growth. Microarray analysis of these and GHR(-/-) mice demonstrated that particular signaling domains are responsible for the regulation of different target genes and revealed novel actions of growth hormone. These mice represent the first step in delineating the domains of the GHR regulating body growth and composition and the transcripts associated with these domains.
Figures


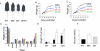
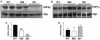

References
-
- Ayling, R. M., R. Ross, P. Towner, S. Von Laue, J. Finidori, S. Moutoussamy, C. R. Buchanan, P. E. Clayton, and M. R. Norman. 1997. A dominant-negative mutation of the growth hormone receptor causes familial short stature. Nat. Genet. 16:13-14. - PubMed
-
- Choi, H. K., and D. J. Waxman. 2000. Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology 141:3245-3255. - PubMed
-
- Colosi, P., K. Wong, S. R. Leong, and W. I. Wood. 1993. Mutational analysis of the intracellular domain of the human growth hormone receptor. J. Biol. Chem. 268:12617-12623. - PubMed
-
- Coschigano, K. T., D. Clemmons, L. L. Bellush, and J. J. Kopchick. 2000. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141:2608-2613. - PubMed
-
- Davey, H. W., T. Xie, M. J. McLachlan, R. J. Wilkins, D. J. Waxman, and D. R. Grattan. 2001. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142:3836-3841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
