Structural and functional analysis of essential pre-mRNA splicing factor Prp19p
- PMID: 15601865
- PMCID: PMC538785
- DOI: 10.1128/MCB.25.1.451-460.2005
Structural and functional analysis of essential pre-mRNA splicing factor Prp19p
Abstract
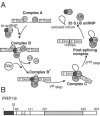
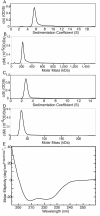
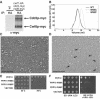
U-box-containing Prp19p is an integral component of the Prp19p-associated complex (the nineteen complex, or NTC) that is essential for activation of the spliceosome. Prp19p makes numerous protein-protein contacts with other NTC components and is required for NTC stability. Here we show that Prp19p forms a tetramer in vitro and in vivo and we map the domain required for its oligomerization to a central tetrameric coiled-coil. Biochemical and in vivo analyses are consistent with Prp19p tetramerization providing an interaction surface for a single copy of its binding partner, Cef1p. Electron microscopy showed that the isolated Prp19p tetramer is an elongated particle consisting of four globular WD40 domains held together by a central stalk consisting of four N-terminal U-boxes and four coiled-coils. These structural and functional data provide a basis for understanding the role of Prp19p as a key architectural component of the NTC.
Figures





References
-
- Andrade, M. A., P. Chacon, J. J. Merelo, and F. Moran. 1993. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 6:383-390. - PubMed
-
- Chan, S. P., D. I. Kao, W. Y. Tsai, and S. C. Cheng. 2003. The Prp19p-associated complex in spliceosome activation. Science 302:279-282. - PubMed
-
- Chen, C. H., W. Y. Tsai, H. R. Chen, C. H. Wang, and S. C. Cheng. 2001. Identification and characterization of two novel components of the Prp19p-associated complex, Ntc30p and Ntc20p. J. Biol. Chem. 276:488-494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
