A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis
- PMID: 15601897
- PMCID: PMC545926
- DOI: 10.1091/mbc.e04-09-0774
A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis
Abstract
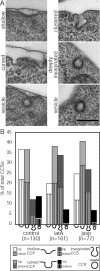
Clathrin-mediated endocytosis in mammalian cells is critical for a variety of cellular processes including nutrient uptake and cell surface receptor down-regulation. Despite the findings that numerous endocytic accessory proteins directly or indirectly regulate actin dynamics and that actin assembly is spatially and temporally coordinated with endocytosis, direct functional evidence for a role of actin during clathrin-coated vesicle formation is lacking. Here, we take parallel biochemical and microscopic approaches to address the contribution of actin polymerization/depolymerization dynamics to clathrin-mediated endocytosis. When measured using live-cell fluorescence microscopy, disruption of the F-actin assembly and disassembly cycle with latrunculin A or jasplakinolide results in near complete cessation of all aspects of clathrin-coated structure (CCS) dynamics. Stage-specific biochemical assays and quantitative fluorescence and electron microscopic analyses establish that F-actin dynamics are required for multiple distinct stages of clathrin-coated vesicle formation, including coated pit formation, constriction, and internalization. In addition, F-actin dynamics are required for observed diverse CCS behaviors, including splitting of CCSs from larger CCSs, merging of CCSs, and lateral mobility on the cell surface. Our results demonstrate a key role for actin during clathrin-mediated endocytosis in mammalian cells.
Figures

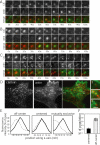
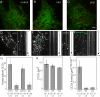
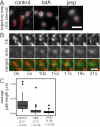
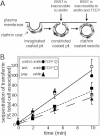

References
-
- Ayscough, K. R. (2000). Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol. 10, 1587-1590. - PubMed
-
- Axelrod, D. (2001). Total internal reflection fluorescence microscopy in cell biology. Traffic 2, 764-774. - PubMed
-
- Bennett, E. M., Chen, C. Y., Engqvist-Goldstein, A. E., Drubin, D. G., and Brodsky, F. M. (2001). Clathrin hub expression dissociates the actin-binding protein Hip1R from coated pits and disrupts their alignment with the actin cytoskeleton. Traffic 2, 851-858. - PubMed
-
- Bubb, M. R., Senderowicz, A. M., Sausville, E. A., Duncan, K. L., and Korn, E. D. (1994). Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269, 14869-14871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

