Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area
- PMID: 15601930
- PMCID: PMC6730354
- DOI: 10.1523/JNEUROSCI.3009-04.2004
Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area
Abstract
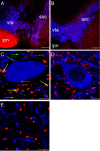
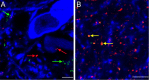
Alpha7 neuronal nicotinic acetylcholine receptors (nAChRs) constitute one of the predominant nAChR subtypes in the mammalian brain. Within the ventral tegmental area (VTA), nicotine application, paired with postsynaptic stimulation, contributes to a form of long-term potentiation, an effect attributed to presynaptic alpha7 nAChRs on glutamatergic afferents (Mansvelder and McGehee, 2000). The aim of this study was to examine the precise subcellular distribution of alpha7 nAChRs in the adult rat VTA to establish whether these receptors are indeed present on glutamatergic axon terminals and to determine their relationship with cholinergic afferents. The spatial relationship between alpha7 nAChRs, labeled using the alpha7 nAChR-specific antagonist alpha-bungarotoxin, and the local neurochemical environment was investigated by the application of multiple labeling strategies with antibodies against tyrosine hydroxylase, vesicular glutamate transporters (VGluTs), vesicular acetylcholine transporter, and glial fibrillary acidic protein. alpha7 nAChRs were localized at both somatodendritic and presynaptic loci within the VTA: on subpopulations of dopaminergic and nondopaminergic neurons and glutamatergic and nonglutamatergic terminals. There was no detectable alpha7 nAChR expression within astrocytes in the VTA. Most alpha7 nAChRs were cytoplasmic (82%), and the remainder were associated with the plasma membrane. Most presynaptic receptors (75%) were on glutamatergic axon terminals, with similar levels of alpha-bungarotoxin binding present on both VGluT1- and VGluT2-immunoreactive boutons. Both preembedding and postembedding electron microscopy revealed that presynaptic alpha7 nAChRs are often located at extrasynaptic (27%) and perisynaptic (61%) loci. alpha7 nAChRs were not associated with cholinergic synapses, consistent with their activation by a paracrine mode of acetylcholine or choline delivery.
Figures






References
-
- Alkondon M, Rocha ES, Maelicke A, Albuquerque EX (1996) Diversity of nicotinic acetylcholine receptors in rat brain. V. α-Bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther 278: 1460-1471. - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX (1997) Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9: 2734-2742. - PubMed
-
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7: 613-620. - PubMed
-
- Caruncho HJ, Guidotti A, Lindstrom J, Costa E, Pesold C (1997) Subcellular localization of the α7 nicotinic receptor in rat cerebellar granule cell layer. NeuroReport 8: 1431-1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
