Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds
- PMID: 15604141
- PMCID: PMC539727
- DOI: 10.1073/pnas.0407817101
Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds
Abstract
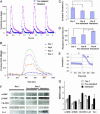
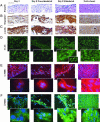
The major challenge of tissue engineering is directing the cells to establish the physiological structure and function of the tissue being replaced across different hierarchical scales. To engineer myocardium, biophysical regulation of the cells needs to recapitulate multiple signals present in the native heart. We hypothesized that excitation-contraction coupling, critical for the development and function of a normal heart, determines the development and function of engineered myocardium. To induce synchronous contractions of cultured cardiac constructs, we applied electrical signals designed to mimic those in the native heart. Over only 8 days in vitro, electrical field stimulation induced cell alignment and coupling, increased the amplitude of synchronous construct contractions by a factor of 7, and resulted in a remarkable level of ultrastructural organization. Development of conductive and contractile properties of cardiac constructs was concurrent, with strong dependence on the initiation and duration of electrical stimulation.
Figures


References
-
- American Heart Association (2003) Heart Disease and Stroke Statistics: 2004 Update (Am. Heart Assoc., Dallas).
-
- Carrier, R. L., Papadaki, M., Rupnick, M., Schoen, F. J., Bursac, N., Langer, R., Freed, L. E. & Vunjak-Novakovic, G. (1999) Biotechnol. Bioeng. 64, 580–589. - PubMed
-
- Eschenhagen, T., Fink, C., Remmers, U., Scholz, H., Wattchow, J., Woil, J., Zimmermann, W., Dohmen, H. H., Schafer, H., Bishopric, N., et al. (1997) FASEB J. 11, 683–694. - PubMed
-
- Leor, J., Aboulafia-Etzion, S., Dar, A., Shapiro, L., Barbash, I. M., Battler, A., Granot, Y. & Cohen, S. (2000) Circulation 102, Suppl. 3, III56–III61. - PubMed
-
- Li, R.-K., Jia, Z. Q., Weisel, R. D., Mickle, D. A. G., Choi, A. & Yau, T. M. (1999) Circulation 100, Suppl., II63–II69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

