Reversal of long-term sepsis-induced immunosuppression by dendritic cells
- PMID: 15604223
- PMCID: PMC1895017
- DOI: 10.1182/blood-2004-08-3251
Reversal of long-term sepsis-induced immunosuppression by dendritic cells
Abstract
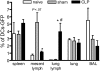
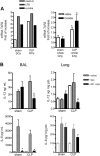
Severe sepsis leads to long-term systemic and local immunosuppression, which is the cause of a number of complications, including pulmonary infection. A therapeutic strategy that reverses this immunosuppression is required, given the ongoing high mortality rate of patients who have survived a severe sepsis. The present study demonstrates that experimental severe sepsis renders the lung susceptible to a normally innocuous Aspergillus fumigatus fungus challenge, due to a dominant lung type 2 cytokine profile. Dendritic cells (DCs) obtained from the lungs of mice subjected to cecal ligation and puncture (CLP) model were skewed toward type 2 cytokine profile, which occurred with exaggerated expression of Toll-like receptor 2 (TLR2). The intrapulmonary transfer of bone marrow-derived DCs (BMDCs) in postseptic mice prevented fatal Aspergillus infection. This therapy reduced the overall inflammatory response and fungal growth in the lung, and promoted the balance of proinflammatory and suppressive cytokines in the lung. Thus, intrapulmonary DC supplementation appears to restore the pulmonary host response in the postseptic lung in our animal model. These data strongly suggest that lung DCs are profoundly affected as a consequence of the systemic impact of severe sepsis, and the identification of mechanisms that restore their function may serve as a key strategy to reverse sepsis-induced immunosuppression.
Figures
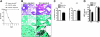


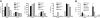
References
-
- Romani L, Kaufmann SH. Immunity to fungi: editorial overview. Res Immunol. 1998;149: 277-281. - PubMed
-
- Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin Immunol. 2004;16: 23-26. - PubMed
-
- Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med. 2001;29: S2-S6; discussion S6-S7. - PubMed
-
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420: 885-891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

