The effect of trimethoprim on CYP2C8 mediated rosiglitazone metabolism in human liver microsomes and healthy subjects
- PMID: 15606443
- PMCID: PMC1884957
- DOI: 10.1111/j.1365-2125.2005.02263.x
The effect of trimethoprim on CYP2C8 mediated rosiglitazone metabolism in human liver microsomes and healthy subjects
Abstract
Aims: Rosiglitazone, a thiazolidinedione antidiabetic medication used in the treatment of Type 2 diabetes mellitus, is predominantly metabolized by the cytochrome P450 (CYP) enzyme CYP2C8. The anti-infective drug trimethoprim has been shown in vitro to be a selective inhibitor of CYP2C8. The purpose of this study was to evaluate the effect of trimethoprim on the CYP2C8 mediated metabolism of rosiglitazone in vivo and in vitro.
Methods: The effect of trimethoprim on the metabolism of rosiglitazone in vitro was assessed in pooled human liver microsomes. The effect in vivo was determined by evaluating rosiglitazone pharmacokinetics in the presence and absence of trimethoprim. Eight healthy subjects (four men and four women) completed a randomized, cross-over study. Subjects received single dose rosiglitazone (8 mg) in the presence and absence of trimethoprim 200 mg given twice daily for 5 days.
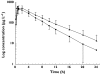
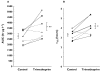
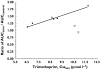
Results: Trimethoprim inhibited rosiglitazone metabolism both in vitro and in vivo. Inhibition of rosiglitazone para-hydroxylation by trimethoprim in vitro was found to be competitive with apparent K(i) and IC(50) values of 29 microm and 54.5 microm, respectively. In the presence of trimethoprim, rosiglitazone plasma AUC was increased by 31% (P = 0.01) from 2774 +/- 645 microg l(-1) h to 3643 +/- 1051 microg l(-1) h (95% confidence interval (CI) for difference 189, 1549), and half-life was increased by 27% (P = 0.006) from 3.3 +/- 0.5 to 4.2 +/- 0.8 h (95% CI for difference 0.36, 1.5). Trimethoprim reduced the para-O-sulphate rosiglitazone/rosiglitazone and the N-desmethylrosiglitazone/rosiglitazone AUC(0-24) ratios by 22% and 38%, respectively.
Conclusions: These results indicate that trimethoprim is a competitive inhibitor of CYP2C8-mediated rosiglitazone metabolism in vitro and that trimethoprim administration increases plasma rosiglitazone concentrations in healthy subjects.
Figures
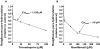
Similar articles
-
Effects of trimethoprim and rifampin on the pharmacokinetics of the cytochrome P450 2C8 substrate rosiglitazone.Clin Pharmacol Ther. 2004 Sep;76(3):239-49. doi: 10.1016/j.clpt.2004.05.001. Clin Pharmacol Ther. 2004. PMID: 15371985 Clinical Trial.
-
Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone.Br J Clin Pharmacol. 1999 Sep;48(3):424-32. doi: 10.1046/j.1365-2125.1999.00030.x. Br J Clin Pharmacol. 1999. PMID: 10510156 Free PMC article.
-
Application of in vitro-in vivo extrapolation (IVIVE) and physiologically based pharmacokinetic (PBPK) modelling to investigate the impact of the CYP2C8 polymorphism on rosiglitazone exposure.Eur J Clin Pharmacol. 2013 Jun;69(6):1311-20. doi: 10.1007/s00228-012-1467-3. Epub 2013 Jan 11. Eur J Clin Pharmacol. 2013. PMID: 23307233
-
Pharmacokinetic interactions with thiazolidinediones.Clin Pharmacokinet. 2007;46(1):1-12. doi: 10.2165/00003088-200746010-00001. Clin Pharmacokinet. 2007. PMID: 17201456 Review.
-
An in-depth review of PPARγ modulators as anti-diabetes therapeutics.Drug Metab Rev. 2025 May 23:1-27. doi: 10.1080/03602532.2025.2508152. Online ahead of print. Drug Metab Rev. 2025. PMID: 40406962 Review.
Cited by
-
The role of pharmacogenetics in drug disposition and response of oral glucose-lowering drugs.Clin Pharmacokinet. 2013 Oct;52(10):833-54. doi: 10.1007/s40262-013-0076-3. Clin Pharmacokinet. 2013. PMID: 23719679 Review.
-
Chemical inhibitors of cytochrome P450 isoforms in human liver microsomes: a re-evaluation of P450 isoform selectivity.Eur J Drug Metab Pharmacokinet. 2011 Mar;36(1):1-16. doi: 10.1007/s13318-011-0024-2. Epub 2011 Feb 19. Eur J Drug Metab Pharmacokinet. 2011. PMID: 21336516 Review.
-
Pharmacogenomics and Personalized Medicine in Type 2 Diabetes Mellitus: Potential Implications for Clinical Practice.Pharmgenomics Pers Med. 2021 Nov 13;14:1441-1455. doi: 10.2147/PGPM.S329787. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 34803393 Free PMC article. Review.
-
Cytochrome P450 2C8 pharmacogenetics: a review of clinical studies.Pharmacogenomics. 2009 Sep;10(9):1489-510. doi: 10.2217/pgs.09.82. Pharmacogenomics. 2009. PMID: 19761371 Free PMC article.
-
Pharmacokinetics and Pharmacodynamics of Ibrexafungerp.Drugs R D. 2022 Mar;22(1):9-13. doi: 10.1007/s40268-021-00376-x. Epub 2021 Dec 27. Drugs R D. 2022. PMID: 34961907 Free PMC article. Review.
References
-
- Klose TS, Blaisdell JA, Goldstein JA. Gene structure of CYP2C8 and extrahepatic distribution of the human CYP2Cs. J Biochem Mol Toxicol. 1999;13(6):289–95. - PubMed
-
- Lapple F, von Richter O, Fromm MF, Richter T, Thon KP, Wisser H, et al. Differential expression and function of CYP2C isoforms in human intestine and liver. Pharmacogenetics. 2003;13(9):565–75. - PubMed
-
- Mace K, Bowman ED, Vautravers P, Shields PG, Harris CC, Pfeifer AM. Characterisation of xenobiotic-metabolising enzyme expression in human bronchial mucosa and peripheral lung tissues. Eur J Cancer. 1998;34(6):914–20. - PubMed
-
- Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, et al. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics. 2001;11(7):597–607. - PubMed
-
- Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D, Dunn SA, Gilissen R, et al. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem Pharmacol. 2002;64(11):1579–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

