Bioavailability of diclofenac potassium at low doses
- PMID: 15606444
- PMCID: PMC1884962
- DOI: 10.1111/j.1365-2125.2005.02226.x
Bioavailability of diclofenac potassium at low doses
Abstract
Aim: Diclofenac-K has been recently launched at low oral doses in different countries for over-the-counter use. However, given the considerable first-pass metabolism of diclofenac, the degree of absorption of diclofenac-K at low doses remained to be determined. The aim of this study was to determine the bioavailability of low-dose diclofenac-K.
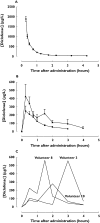
Methods: A randomized, three-way, cross-over study was performed in 10 subjects. Each received diclofenac-K, 22.5 mg via short-term i.v. infusion and orally at single doses of 12.5 mg and 25 mg.
Results: Mean (+/- SD) times to maximal plasma concentration (t(max)) of diclofenac were 0.48 +/- 0.28 h (12.5 mg) and 0.93 +/- 0.96 h (25 mg). The absolute bioavailability of diclofenac-K after oral administration did not differ significantly in the 12.5-mg and 25-mg dose group (63.1 +/- 12.6%vs. 65.1 +/- 19.4%, respectively). The 90% confidence intervals for the AUC(infinity) and AUC(t) ratios for the two oral regimes were 82.6, 103.4% (point estimate 92.4%) and 86.2, 112.9% (point estimate 98.6%), respectively. These values were within the acceptance criteria for bioequivalence (80-125%).
Conclusions: Our data indicate that diclofenac-K is rapidly and well absorbed at low dose, and are consistent with a rapid onset of action of the drug. Abbreviations AUC, area under plasma concentration-time curve; C(max), peak plasma concentration; CI, confidence interval; COX, cyclooxygenase; D, dose; F, absolute bioavailability; t(max), time to reach C(max).
Figures
References
-
- Hinz B, Rau T, Auge D, et al. Aceclofenac spares cyclooxygenase 1 as a result of limited but sustained biotransformation to diclofenac. Clin Pharmacol Ther. 2003;74:222–35. - PubMed
-
- Brune K, Lanz R. Pharmacokinetics of non-steroidal anti-inflammatory drugs. In: Bonta IL, Bray MA, Parnham MJ, editors. Handbook of Inflammation, Vol. 5, The Pharmacology of Inflammation. Amsterdam: Elsevier; 1985. pp. 413–49.
-
- John VA. The pharmacokinetics and metabolism of diclofenac sodium (Voltarol) in animals and man. Rheumatol Rehabil Suppl. 1979;2:22–37. - PubMed
-
- Willis JV, Kendall MJ, Flinn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol. 1979;16:405–10. - PubMed
-
- McNeely W, Goa KL. Diclofenac-potassium in migraine: a review. Drugs. 1999;57:991–1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

