Phenotypic and functional heterogeneity of porcine blood monocytes and its relation with maturation
- PMID: 15606796
- PMCID: PMC1782062
- DOI: 10.1111/j.1365-2567.2004.01994.x
Phenotypic and functional heterogeneity of porcine blood monocytes and its relation with maturation
Abstract
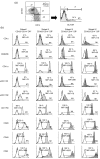
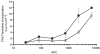
Swine monocytes constitute a heterogeneous population of cells which can be divided into four subsets based on the expression of SWC3, CD14, CD163 and swine leucocyte antigen (SLA) DR markers. These subsets appear to represent different maturation stages in a pathway along which these cells up-regulate the expression of SLA DR and CD163 antigens and reduce that of CD14. Differences in the expression of adhesion and costimulatory molecules are also patent, with a progressive increase in the expression of CD11a, wCD11R1, CD29, CD49d, CD61, CD1a and CD80/86, and a concomitant decrease in that of wCD11R2. Besides, these subsets differ in their capacity for tumour necrosis factor-alpha (TNF-alpha) production in response to lipopolysaccharide + interferon-gamma. The CD163(+) CD14(-) SLA DR(+) subset produces higher amounts of TNF-alpha than the CD163(-) CD14(+) SLA DR(-) subset, whereas CD163(+) CD14(+) SLA DR(+) and CD163(-) CD14(+) SLA DR(+) subsets show intermediate values. CD163(+) monocytes also display a higher ability to present soluble antigens to T cells than CD163(-) monocytes.
Figures


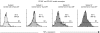
References
-
- Gordon S, Keshav S, Chung LP. Mononuclear phagocytes: tissue distribution and functional heterogeneity. Curr Opin Immunol. 1988;1:26–35. - PubMed
-
- Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997;27:431–41. - PubMed
-
- Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–95. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

