Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis
- PMID: 15608336
- PMCID: PMC544504
- DOI: 10.1105/tpc.104.026971
Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis
Abstract
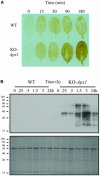
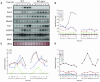
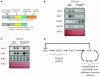
Reactive oxygen species (ROS), such as O2- and H2O2, play a key role in plant metabolism, cellular signaling, and defense. In leaf cells, the chloroplast is considered to be a focal point of ROS metabolism. It is a major producer of O2- and H2O2 during photosynthesis, and it contains a large array of ROS-scavenging mechanisms that have been extensively studied. By contrast, the function of the cytosolic ROS-scavenging mechanisms of leaf cells is largely unknown. In this study, we demonstrate that in the absence of the cytosolic H2O2-scavenging enzyme ascorbate peroxidase 1 (APX1), the entire chloroplastic H2O2-scavenging system of Arabidopsis thaliana collapses, H2O2 levels increase, and protein oxidation occurs. We further identify specific proteins oxidized in APX1-deficient plants and characterize the signaling events that ensue in knockout-Apx1 plants in response to a moderate level of light stress. Using a dominant-negative approach, we demonstrate that heat shock transcription factors play a central role in the early sensing of H2O2 stress in plants. Using knockout plants for the NADPH oxidase D protein (knockout-RbohD), we demonstrate that RbohD might be required for ROS signal amplification during light stress. Our study points to a key role for the cytosol in protecting the chloroplast during light stress and provides evidence for cross-compartment protection of thylakoid and stromal/mitochondrial APXs by cytosolic APX1.
Figures


References
-
- Akenov, M.Y., Aksenova, M.V., Butterfield, D.A., Geddes, J.W., and Markesbery, W.R. (2001). Protein oxidation in the brain in Alzheimer's disease. Neuroscience 103, 373–383. - PubMed
-
- Apel, K., and Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. - PubMed
-
- Asada, K. (1999). The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

