Cross-typic specificity and immunotherapeutic potential of a human HPV16 E7-specific CTL line
- PMID: 15609329
- PMCID: PMC7165518
- DOI: 10.1002/ijc.20779
Cross-typic specificity and immunotherapeutic potential of a human HPV16 E7-specific CTL line
Abstract
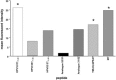
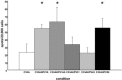
Cervical cancer (CaCx) is strongly associated with human papillomavirus (HPV) infection, particularly HPV types 16 and 18. The constitutive expression of HPV E6 and E7 proteins in CaCx makes them attractive targets for CTL based immunotherapy. However cervical carcinomas may have features, e.g., antigen processing defects, that limit the effectiveness of HPV specific CTL. Furthermore most vaccine development has concentrated on HPV type 16, and it is not clear whether such vaccines could induce CTL able to cross-react on related oncogenic HPV types, e.g., HPV31 and 52. To investigate these potentially important parameters in vitro, we used a CTL (D4) specific for HPV16 E7(11-20). D4 was able to kill a variety of HPV16+ CaCx cell lines including those with suspected (CaSki) or known antigen processing defects (C33A), and with low HPV DNA copy number (SiHa). D4 was also able to cross react on a related peptide from HPV52 E7 but not HPV31 E7. Further analysis suggested that D4 cross reactivity against related peptides was influenced both by TCR contact residues and a certain threshold for peptide binding. The HPV cross-reactivity was confirmed at the whole protein level as D4 was also able to recognize the endogenously processed forms of HPV16 and 52 E7 but not 31 E7. These results suggest that HPV16 E7(11-20) would be a useful epitope for immunotherapy in both HPV 16 and 52 tumours. Despite this, it is difficult to generate these CTL in response to vaccination, emphasizing the need for definition of novel epitopes and more efficient vaccination strategies.
Figures



References
-
- Parkin DM. The global burden of cancer. Semin Cancer Biol 1998; 8: 219–35. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meiher CJ, Munzo N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–19. - PubMed
-
- Vousden K. Interactions between papillomavirus proteins and tumor suppressor gene products. Adv Cancer Res 1994; 64: 1–24. - PubMed
-
- Munoz N, Bosch X, Sanjose Sd, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiological classification of human papillomavirus types associated with cervical cancer. New Engl J Med 2003; 348: 518–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

