In vitro activity of antiamoebic drugs against clinical isolates of Entamoeba histolytica and Entamoeba dispar
- PMID: 15610563
- PMCID: PMC544836
- DOI: 10.1186/1476-0711-3-27
In vitro activity of antiamoebic drugs against clinical isolates of Entamoeba histolytica and Entamoeba dispar
Abstract
Background: Amoebiasis is a major public health problem in tropical and subtropical countries. Although a number of antiamoebic agents are used for its treatment, yet the susceptibility data on clinical isolates of Entamoeba histolytica and Entamoeba dispar are not available. Therefore, the present study was aimed to assess the in vitro susceptibility of clinical isolates of E. histolytica and E. dispar to metronidazole, chloroquine, emetine and tinidazole.
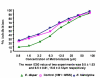
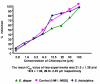
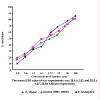
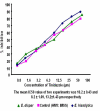
Methods: A total of 45 clinical isolates (15 E. histolytica and 30 E. dispar) were maintained in polyxenic cultures followed by monoxenic cultures. In vitro drug sensitivity (IC50) of clinical isolates and standard reference strain of E. histolytica (HM1: IMSS) was assessed by nitro blue tetrazolium (NBT) reduction assay after exposure to various concentrations of each drug.
Results: The results showed that all clinical isolates had a higher IC50 compared to reference strain to all the four drugs. E. histolytica isolates appeared to be more susceptible [IC50 (microm) 13.2,26.3,31.2 and 12.4] compared to E. dispar isolates [IC50(microm) 15.6,28.9,32.8 and 13.2] and the reference strain of E. histolytica [IC50 (microm) 9.5, 15.5, 29.9 and 10.2] to the metronidazole, chloroquine, emetine and tinidazole respectively.
Conclusions: The results indicate that till date, Entamoeba isolates in India do not seem to be resistant to the commonly used antiamoebic drugs.
Figures
References
-
- Walsh JA. Problems in recognition and diagnosis of amoebiasis: estimation of the global magnitude of mortality and morbidity. Rev Infect Dis. 1986;8:228–38. - PubMed
-
- Diamond LS, Clark CG. A redescription of Entamoeba histolytica Schaudinn 1903 (emended walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol. 1993;40:340–4. - PubMed
-
- Martinez-Palomo A, Martinez-Baez M. Selective primary health care: strategies for control of disease in the developing world X amoebiasis. Rev Infect Dis. 1983;5:1093–1102. - PubMed
-
- Gomez MD, Perez DG, Ayala P, Samuelson J, Orozco E. Physiology and molecular biology of multidrug resistance in Entamoeba histolytica. Arch Med Res. 1996;27:421–25. - PubMed
-
- Pittman FE, Pittman JC. Amoebic liver abscess following metronidazole therapy for amoebic colitis. Am J Trop Med Hyg. 1974;23:146–50. - PubMed
LinkOut - more resources
Full Text Sources

