Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation
- PMID: 15611238
- PMCID: PMC2903964
- DOI: 10.4049/jimmunol.174.1.164
Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation
Abstract
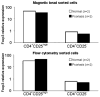
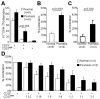
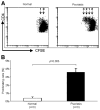
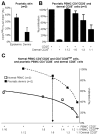
The balance between regulatory and effector functions is important for maintaining efficient immune responses, while avoiding autoimmunity. The inflammatory skin disease psoriasis is sustained by the ongoing activation of pathogenic effector T cells. We found that a CD4(+) T lymphocyte subpopulation in peripheral blood, phenotypically CD25(high), CTLA-4(+), Foxp3(high) (regulatory T (Treg) cells), is deficient in its suppressor activity in psoriasis. This was associated with accelerated proliferation of CD4(+) responder T cells in psoriasis, the majority of which expressed CXCR3. Nevertheless, criss-cross experiments isolated the defect to psoriatic Treg cells. To examine Treg cells in a nonlymphoid tissue of a human T cell-mediated disease, Treg cells were also analyzed and isolated from the site of inflammation, psoriatic lesional skin. At the regulatory vs effector T cells ratios calculated to be present in skin, however, the psoriatic Treg cell population demonstrated decreased suppression of effector T cells. Thus, dysfunctional blood and target tissue CD4(+)CD25(high) Treg cell activity may lead to reduced restraint and consequent hyperproliferation of psoriatic pathogenic T cells in vivo. These findings represent a critical component of human organ-specific autoimmune disease and may have important implications with regard to the possible therapeutic manipulation of Treg cells in vivo.
Figures





References
-
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816. - PubMed
-
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969. - PubMed
-
- Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

