Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease
- PMID: 15611290
- PMCID: PMC2211993
- DOI: 10.1084/jem.20040890
Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease
Abstract
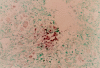
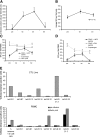
Epstein Barr virus (EBV)+ Hodgkin's disease (HD) expresses clearly identified tumor antigens derived from the virus and could, in principle, be a target for adoptive immunotherapy with viral antigen-specific T cells. However, like most tumor-associated antigens in immunocompetent hosts, these potential targets are only weakly immunogenic, consisting primarily of the latent membrane protein (LMP)1 and LMP2 antigens. Moreover, Hodgkin tumors possess a range of tumor evasion strategies. Therefore, the likely value of immunotherapy with EBV-specific cytotoxic effector cells has been questioned. We have now used a combination of gene marking, tetramer, and functional analyses to track the fate and assess the activity of EBV cytotoxic T lymphocyte (CTL) lines administered to 14 patients treated for relapsed EBV+ HD. Gene marking studies showed that infused effector cells could further expand by several logs in vivo, contribute to the memory pool (persisting up to 12 mo), and traffic to tumor sites. Tetramer and functional analyses showed that T cells reactive with the tumor-associated antigen LMP2 were present in the infused lines, expanded in peripheral blood after infusion, and also entered tumor. Viral load decreased, demonstrating the biologic activity of the infused CTLs. Clinically, EBV CTLs were well tolerated, could control type B symptoms (fever, night sweats, and weight loss), and had antitumor activity. After CTL infusion, five patients were in complete remission at up to 40 mo, two of whom had clearly measurable tumor at the time of treatment. One additional patient had a partial response, and five had stable disease. The performance and fate of these human tumor antigen-specific T cells in vivo suggests that they might be of value for the treatment of EBV+ Hodgkin lymphoma.
Figures
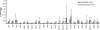
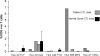





References
-
- Rooney, C.M., C.A. Smith, C. Ng, S.K. Loftin, C. Li, R.A. Krance, M.K. Brenner, and H.E. Heslop. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 345:9–13. - PubMed
-
- Heslop, H.E., C.Y.C. Ng, C. Li, C.A. Smith, S.K. Loftin, R.A. Krance, M.K. Brenner, and C.M. Rooney. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2:551–555. - PubMed
-
- Rooney, C.M., C.A. Smith, C.Y.C. Ng, S.K. Loftin, J.W. Sixbey, Y.-J. Gan, D.-K. Srivastava, L.C. Bowman, R.A. Krance, M.K. Brenner, and H.E. Heslop. 1998. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 92:1549–1555. - PubMed
-
- Gustafsson, A., V. Levitsky, J.Z. Zou, T. Frisan, T. Dalianis, P. Ljungman, O. Ringden, J. Winiarski, I. Ernberg, and M.G. Masucci. 2000. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 95:807–814. - PubMed
-
- O'Reilly, R.J., T.N. Small, E. Papadopoulos, K. Lucas, J. Lacerda, and L. Koulova. 1998. Adoptive immunotherapy for Epstein-Barr virus-associated lymphoproliferative disorders complicating marrow allografts. Springer Semin. Immunopathol. 20:455–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

