Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists
- PMID: 15611291
- PMCID: PMC2211988
- DOI: 10.1084/jem.20041215
Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists
Abstract
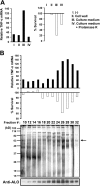
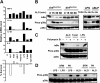
Exposure of bone marrow-derived macrophages (BMDMs) to low concentrations of Bacillus anthracis lethal toxin (LT), whose catalytic subunit is lethal factor (LF), results in induction of a robust apoptotic response dependent on activation of Toll-like receptor (TLR)4. A similar TLR4-dependent apoptotic response is observed when BMDMs are infected with live B. anthracis (Sterne strain). However, TLR4 is considered to be a specific signaling receptor for lipopolysaccharide (LPS), a typical product of gram-negative bacteria, whereas B. anthracis is gram-positive. To understand how B. anthracis can activate TLR4, we analyzed its culture supernatants and found them to contain a potent TLR4-stimulating activity that can also induce apoptosis in macrophages in which the antiapoptotic p38 MAP kinase (whose activation is prevented by LF) was inhibited. Purification of this activity suggested it consists of anthrolysin O (ALO), a member of the cholesterol-dependent cytolysin (CDC) family. We show that recombinant ALO can activate TLR4 in a manner independent of LPS contamination and, together with LT, can induce macrophage apoptosis. We also provide genetic evidence that ALO is required for induction of macrophage apoptosis in response to infection with live B. anthracis and that other CDC family members share the ability to activate TLR4.
Figures




References
-
- Dixon, T.C., M. Meselson, J. Guillemin, and P.C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815–826. - PubMed
-
- Collier, R.J., and J.A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45–70. - PubMed
-
- Moayeri, M., and S.H. Leppla. 2004. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 7:19–24. - PubMed
-
- Bradley, K.A., J. Mogridge, M. Mourez, R.J. Collier, and J.A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature. 414:225–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

