Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes
- PMID: 15611293
- PMCID: PMC2211995
- DOI: 10.1084/jem.20041545
Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes
Abstract
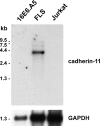
Cadherins are integral membrane proteins expressed in tissue-restricted patterns that mediate homophilic intercellular adhesion. During development, they orchestrate tissue morphogenesis and, in the adult, they determine tissue integrity and architecture. The synovial lining is a condensation of fibroblast-like synoviocytes (FLS) and macrophages one to three cells thick. These cells are embedded within the extracellular matrix, but the structure is neither an epithelium nor an endothelium. Previously, the basis for organization of the synovium into a tissue was unknown. Here, we cloned cadherin-11 from human rheumatoid arthritis (RA)-derived FLS. We developed L cell transfectants expressing cadherin-11, cadherin-11 fusion proteins, and anti-cadherin-11 mAb. Cadherin-11 was found to be expressed mainly in the synovial lining by immunohistologic staining of human synovium. FLS adhered to cadherin-11-Fc, and transfection of cadherin-11 conferred the formation of tissue-like sheets and lining-like structures upon fibroblasts in vitro. These findings support a key role for cadherin-11 in the specific adhesion of FLS and in synovial tissue organization and behavior in health and RA.
Figures




References
-
- Gumbiner, B.M. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 84:345–357. - PubMed
-
- Wheelock, M.J., and K.R. Johnson. 2003. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19:207–235. - PubMed
-
- Perl, A.K., P. Wilgenbus, U. Dahl, H. Semb, and G. Christofori. 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 392:190–193. - PubMed
-
- Pishvaian, M.J., C.M. Feltes, P. Thompson, M.J. Bussemakers, J.A. Schalken, and S.W. Byers. 1999. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 59:947–952. - PubMed
-
- Tomita, K., A. van Bokhoven, G.J. van Leenders, E.T. Ruijter, C.F. Jansen, M.J. Bussemakers, and J.A. Schalken. 2000. Cadherin switching in human prostate cancer progression. Cancer Res. 60:3650–3654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

