The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2
- PMID: 15611342
- PMCID: PMC2172602
- DOI: 10.1083/jcb.200404160
The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2
Abstract
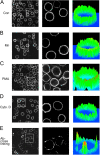
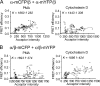
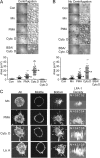
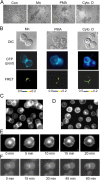
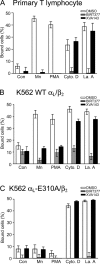
Dynamic regulation of integrin adhesiveness is required for immune cell-cell interactions and leukocyte migration. Here, we investigate the relationship between cell adhesion and integrin microclustering as measured by fluorescence resonance energy transfer, and macroclustering as measured by high resolution fluorescence microscopy. Stimuli that activate adhesion through leukocyte function-associated molecule-1 (LFA-1) failed to alter clustering of LFA-1 in the absence of ligand. Binding of monomeric intercellular adhesion molecule-1 (ICAM-1) induced profound changes in the conformation of LFA-1 but did not alter clustering, whereas binding of ICAM-1 oligomers induced significant microclustering. Increased diffusivity in the membrane by cytoskeleton-disrupting agents was sufficient to drive adhesion in the absence of affinity modulation and was associated with a greater accumulation of LFA-1 to the zone of adhesion, but redistribution did not precede cell adhesion. Disruption of conformational communication within the extracellular domain of LFA-1 blocked adhesion stimulated by affinity-modulating agents, but not adhesion stimulated by cytoskeleton-disrupting agents. Thus, LFA-1 clustering does not precede ligand binding, and instead functions in adhesion strengthening after binding to multivalent ligands.
Figures



References
-
- Bazzoni, G., and M.E. Hemler. 1998. Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci. 23:30–34. - PubMed
-
- Beglova, N., S.C. Blacklow, J. Takagi, and T.A. Springer. 2002. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 9:282–287. - PubMed
-
- Buensuceso, C., M. De Virgilio, and S.J. Shattil. 2003. Detection of integrin αIIbβ3 clustering in living cells. J. Biol. Chem. 278:15217–15224. - PubMed
-
- Carman, C.V., and T.A. Springer. 2003. Integrin avidity regulation: Are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15:547–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

