Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White syndrome
- PMID: 15611370
- PMCID: PMC2908313
- DOI: 10.1161/01.CIR.0000151291.32974.D5
Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White syndrome
Abstract
Background: We identified a gene (PRKAG2) that encodes the gamma-2 regulatory subunit of AMP-activated protein kinase (AMPK) with a mutation (Arg302Gln) responsible for familial Wolff-Parkinson-White (WPW) syndrome. The human phenotype consists of ventricular preexcitation, conduction abnormalities, and cardiac hypertrophy.
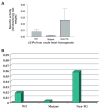
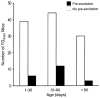
Methods and results: To elucidate the molecular basis for the phenotype, transgenic mice were generated by cardiac-restricted expression of the wild-type (TG(WT)) and mutant(TG(R302Q)) PRKAG2 gene with the cardiac-specific promoter alpha-myosin heavy chain. ECG recordings and intracardiac electrophysiology studies demonstrated the TG(R302Q) mice to have ventricular preexcitation (PR interval 10+/-2 versus 33+/-5 ms in TG(WT), P<0.05) and a prolonged QRS (20+/-5 versus 10+/-1 ms in TG(WT), P<0.05). A distinct AV accessory pathway was confirmed by electrical and pharmacological stimulation and substantiated by induction of orthodromic AV reentrant tachycardia. Enzymatic activity of AMPK in the mutant heart was significantly reduced (0.009+/-0.003 versus 0.025+/-0.001 nmol x min(-1) x g(-1) in nontransgenic mice), presumably owing to the mutation disrupting the AMP binding site. Excessive cardiac glycogen was observed. Hypertrophy was confirmed by increases in heart weight (296 versus 140 mg in TG(WT)) and ventricular wall thickness.
Conclusions: We have developed a genetic animal model of WPW that expresses a mutation responsible for a familial form of WPW syndrome with a phenotype identical to that of the human, including induction of supraventricular arrhythmia. The defect is due to loss of function of AMPK. Elucidation of the molecular basis should provide insight into development of the cardiac conduction system and accessory pathways.
Figures







References
-
- Dunnigan A. Developmental aspects and natural history of preexcitation syndromes. In: Benditt DG, Benson DW, editors. Cardiac Preexcitation Syndromes: Origins, Evaluation and Treatment. Boston, Mass: Martinus Nighoff; 1986. pp. 21–29.
-
- Wan W, Wu N, Fan W, Tang YY, Jin L, Fang Q. Clinical manifestations and prevalence of different types of supraventricular tachycardia among Chinese. Chin Med J. 1992;105:284–288. - PubMed
-
- Bauernfeind RA, Wyndham CR, Swiryn SP, Palileo EV, Strasberg B, Lam W, Westveer D, Rosen KM. Paroxysmal atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1981;47:562–569. - PubMed
-
- James TN. Normal and abnormal consequences of apoptosis in the human heart: from postnatal morphogenesis to paroxysmal arrhythmias. Circulation. 1994;90:556–573. - PubMed
-
- Gollob MH, Green MS, Tang A, Ahmad F, Hassan A, Gollob T, Lozado R, Shah G, Tapscott T, Karibe A, Begley D, Mohiddin S, Fananapazir L, Bachinski L, Roberts R. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

