Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins
- PMID: 15613305
- PMCID: PMC538538
- DOI: 10.1128/JVI.79.2.771-779.2005
Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins
Abstract
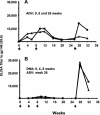
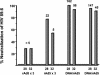
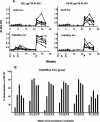
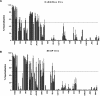
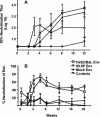
Immunization with recombinant serotype 5 adenoviral (rAd5) vectors or a combination of DNA plasmid priming and rAd5 boosting is known to elicit potent immune responses. However, little data exist regarding these immunization strategies and the development of anti-human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies. We used DNA plasmids and rAd5 vectors encoding the HIV-1 89.6P or chimeric HxB2/BaL envelope glycoprotein to immunize macaque monkeys. A single rAd5 immunization elicited anti-Env antibody responses, but there was little boosting with subsequent rAd5 immunizations. In contrast, rAd5 boosting of DNA-primed monkeys resulted in a rapid rise in antibody titers, including the development of anti-HIV-1 neutralizing antibodies. The potency and breadth of neutralization were evaluated by testing plasma against a panel of 14 clade B primary isolates. Moderate levels of plasma neutralizing activity were detected against about one-third of the viruses tested, and immunoglobulin G fractionation demonstrated that virus neutralization was antibody mediated. After a challenge with a chimeric simian-human immunodeficiency virus (SHIV89.6P), an anamnestic neutralizing antibody response was observed, although the breadth of the response was limited to the subset of viruses that were neutralized after the primary immunization. These data are the first detailed description of the anti-HIV-1 neutralizing antibody response in nonhuman primates elicited by DNA and rAd5 immunization. In addition to the well-established ability of DNA priming and rAd5 boosting to elicit potent anti-HIV-1 cellular immune responses, this immunization strategy elicits anti-HIV-1 neutralizing antibodies and therefore can be used to study novel Env immunogens designed to elicit more potent neutralizing antibodies.
Figures


References
-
- Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. - PubMed
-
- Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

