Virion proteins of Kaposi's sarcoma-associated herpesvirus
- PMID: 15613308
- PMCID: PMC538588
- DOI: 10.1128/JVI.79.2.800-811.2005
Virion proteins of Kaposi's sarcoma-associated herpesvirus
Abstract
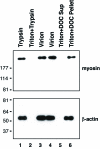
The proteins that compose a herpesvirus virion are thought to contain the functional information required for de novo infection, as well as virion assembly and egress. To investigate functional roles of Kaposi's sarcoma-associated herpesvirus (KSHV) virion proteins in viral productive replication and de novo infection, we attempted to identify virion proteins from purified KSHV by a proteomic approach. Extracellular KSHV virions were purified from phorbol-12-tetradecanoate-13-acetate-induced BCBL-1 cells through double-gradient ultracentrifugation, and their component proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Thirty prominent protein bands were excised and subjected to high-performance liquid chromatography ion trap mass spectrometric analysis. This study led to the identification of 24 virion-associated proteins. These include five capsid proteins, eight envelope glycoproteins, six tegument proteins, and five proteins whose locations in the virions have not yet been defined. Putative tegument proteins encoded by open reading frame 21 (ORF21), ORF33, and ORF45 were characterized and found to be resistant to protease digestion when purified virions were treated with trypsin, confirming that they are located within the virion particles. The ORF64-encoded large tegument protein was found to be associated with capsid but sensitive to protease treatment, suggesting its unique structure and array in KSHV virions. In addition, cellular beta-actin and class II myosin heavy chain type A were found inside KSHV virions and associated with tegument-capsid structure. Identification of KSHV virion proteins makes it possible to study the functional roles of these virion proteins in KSHV replication and pathogenicity.
Figures



Similar articles
-
The ORF45 protein of Kaposi's sarcoma-associated herpesvirus is associated with purified virions.J Virol. 2003 Apr;77(7):4221-30. doi: 10.1128/jvi.77.7.4221-4230.2003. J Virol. 2003. PMID: 12634379 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Inhibitor of cGAS (KicGAS), Encoded by ORF52, Is an Abundant Tegument Protein and Is Required for Production of Infectious Progeny Viruses.J Virol. 2016 May 12;90(11):5329-5342. doi: 10.1128/JVI.02675-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009954 Free PMC article.
-
The Interaction between Tegument Proteins ORF33 and ORF45 Plays an Essential Role in Cytoplasmic Virion Maturation of a Gammaherpesvirus.J Virol. 2022 Nov 23;96(22):e0107322. doi: 10.1128/jvi.01073-22. Epub 2022 Oct 27. J Virol. 2022. PMID: 36300940 Free PMC article.
-
Role of tegument proteins in herpesvirus assembly and egress.Protein Cell. 2010 Nov;1(11):987-98. doi: 10.1007/s13238-010-0120-0. Epub 2010 Dec 10. Protein Cell. 2010. PMID: 21153516 Free PMC article. Review.
-
Betaherpesvirus Virion Assembly and Egress.Adv Exp Med Biol. 2018;1045:167-207. doi: 10.1007/978-981-10-7230-7_9. Adv Exp Med Biol. 2018. PMID: 29896668 Review.
Cited by
-
Analysis of virion-incorporated host proteins required for herpes simplex virus type 1 infection through a RNA interference screen.PLoS One. 2013;8(1):e53276. doi: 10.1371/journal.pone.0053276. Epub 2013 Jan 3. PLoS One. 2013. PMID: 23301054 Free PMC article.
-
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells.J Virol. 2015 Mar;89(6):3093-111. doi: 10.1128/JVI.02507-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552714 Free PMC article.
-
Further evidence of Chelonid herpesvirus 5 (ChHV5) latency: high levels of ChHV5 DNA detected in clinically healthy marine turtles.PeerJ. 2016 Jul 27;4:e2274. doi: 10.7717/peerj.2274. eCollection 2016. PeerJ. 2016. PMID: 27547576 Free PMC article.
-
Tracking expression and subcellular localization of RNA and protein species using high-throughput single cell imaging flow cytometry.RNA. 2012 Aug;18(8):1573-9. doi: 10.1261/rna.033126.112. Epub 2012 Jun 28. RNA. 2012. PMID: 22745225 Free PMC article.
-
Activation and degradation of open reading frame 45 by the replication and transcription activator of Kaposi's sarcoma-associated herpesvirus.J Gen Virol. 2015 Jul;96(Pt 7):1883-9. doi: 10.1099/vir.0.000125. Epub 2015 Mar 17. J Gen Virol. 2015. PMID: 25783474 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

