Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo
- PMID: 15613356
- PMCID: PMC538585
- DOI: 10.1128/JVI.79.2.1296-1307.2005
Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo
Abstract
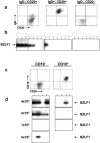
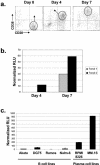
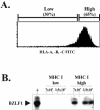
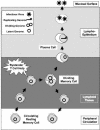
In this paper we demonstrate that the cells which initiate replication of Epstein-Barr virus (EBV) in the tonsils of healthy carriers are plasma cells (CD38hi, CD10-, CD19+, CD20lo, surface immunoglobulin negative, and cytoplasmic immunoglobulin positive). We further conclude that differentiation into plasma cells, and not the signals that induce differentiation, initiates viral replication. This was confirmed by in vitro studies showing that the promoter for BZLF1, the gene that begins viral replication, becomes active only after memory cells differentiate into plasma cells and is also active in plasma cell lines. This differs from the reactivation of BZLF1 in vitro, which occurs acutely and is associated with apoptosis and not with differentiation. We suggest that differentiation and acute stress represent two distinct pathways of EBV reactivation in vivo. The fraction of cells replicating the virus decreases as the cells progress through the lytic cycle such that only a tiny fraction actually release infectious virus. This may reflect abortive replication or elimination of cells by the cellular immune response. Consistent with the later conclusion, the cells did not down regulate major histocompatibility complex class I molecules, suggesting that this is not an immune evasion tactic used by EBV and that the cells remain vulnerable to cytotoxic-T-lymphocyte attack.
Figures



References
-
- Anagnostopoulos, I., M. Hummel, C. Kreschel, and H. Stein. 1995. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood 85:744-750. - PubMed
-
- Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. - PubMed
-
- Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

