Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells
- PMID: 15613377
- PMCID: PMC1665557
- DOI: 10.1113/jphysiol.2004.074740
Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells
Abstract
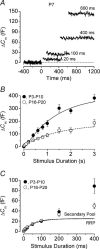
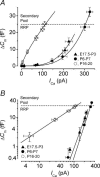
Developmental changes in the coupling between Ca2+ entry and exocytosis were studied in mouse inner hair cells (IHCs) which, together with the afferent endings, form the primary synapse of the mammalian auditory system. Ca2+ currents (ICa) and changes in membrane capacitance (DeltaCm) were recorded using whole-cell voltage clamp from cells maintained at body temperature, using physiological (1.3 mM) extracellular Ca2+. The magnitudes of both ICa and DeltaCm increased with maturation from embryonic stages until postnatal day 6 (P6). Subsequently, ICa gradually declined to a steady level of about -100 pA from P13 while the Ca2+-induced DeltaCm remained relatively constant, indicating a developmental increase in the Ca2+ efficiency of exocytosis. Although the size of ICa changed during development, its activation properties did not, suggesting the presence of a homogeneous population of Ca2+ channels in IHCs throughout development. The Ca2+ dependence of exocytosis changed with maturation from a fourth power relation in immature cells to an approximately linear relation in mature cells. This change applies to the release of both a readily releasable pool (RRP) and a slower secondary pool of vesicles, implying a common release mechanism for these two kinetically distinct pools that becomes modified during development. The increased Ca2+ efficiency and linear Ca2+ dependence of mature IHC exocytosis, especially over the physiological range of intracellular Ca2+, could improve the high-fidelity transmission of both brief and long-lasting stimulation. These properties make the mature cell ideally suited for fine intensity discrimination over a wide dynamic range.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

