The photophysics of green fluorescent protein: influence of the key amino acids at positions 65, 203, and 222
- PMID: 15613627
- PMCID: PMC1305246
- DOI: 10.1529/biophysj.104.044412
The photophysics of green fluorescent protein: influence of the key amino acids at positions 65, 203, and 222
Abstract
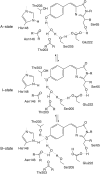
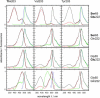
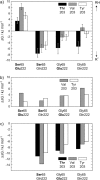
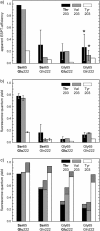
The three amino acids S65, T203, and E222 crucially determine the photophysical behavior of wild-type green fluorescent protein. We investigate the impact of four point mutations at these positions and their respective combinations on green fluorescent protein's photophysics using absorption spectroscopy, as well as steady-state and time-resolved fluorescence spectroscopy. Our results highlight the influence of the protein's hydrogen-bonding network on the equilibrium between the different chromophore states and on the efficiency of the excited-state proton transfer. The mutagenic approach allows us to separate different mechanisms responsible for fluorescence quenching, some of which were previously discussed theoretically. Our results will be useful for the development of new strategies for the generation of autofluorescent proteins with specific photophysical properties. One example presented here is a variant exhibiting uncommon blue fluorescence.
Figures


References
-
- Bae, J. H., M. Rubini, G. Jung, G. Wiegand, M. H. J. Seifert, M. K. Azim, J. S. Kim, A. Zumbusch, T. Holak, L. Moroder, R. Huber, and N. Budisa. 2003. Expansion of the genetic code enables design of a novel “gold” class of green fluorescent proteins. J. Mol. Biol. 328:1071–1081. - PubMed
-
- Baffour-Awuah, N., and M. Zimmer. 2004. Hula-twisting in green fluorescent protein. Chem. Phys. 303:7–11.
-
- Bell, A. F., D. Stoner-Ma, R. M. Wachter, and P. J. Tonge. 2003. Light-driven decarboxylation of wild-type green fluorescent protein. J. Am. Chem. Soc. 125:6919–6926. - PubMed
-
- Branchini, B. R., A. R. Nemser, and M. Zimmer. 1998. A computational analysis of the unique protein-induced tight turn that results in posttranslational chromophore formation in green fluorescent protein. J. Am. Chem. Soc. 120:1–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

